Many patients, particularly those on immunosuppressive medicines, are adversely affected by coronavirus disease 2019 (COVID-19). In addition, substantial evidence suggests that mRNA-based vaccinations generate poorer responses in immunocompromised patients, particularly those receiving anti-CD20 therapy.
Initially, the RituxiVac trial found that only 49% of individuals with kidney transplants, autoimmune diseases and cancer elicit humoral immune response after receiving severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) messenger ribonucleic acid (mRNA) immunization.
At present, there is currently insufficient clinical outcome data to ascertain whether completely vaccinated, but seronegative patients are at least partially protected from severe COVID-19. Further, the time limit for protection against severe COVID-19 by SARS-CoV-2 vaccinations is being debated but considerably more so in immunocompromised persons. Moreover, the antibody trajectories in both healthy and immunocompromised individuals remain unknown.
 Study: Longitudinal analysis of antibody trajectories and humoral responses to a third dose of mRNA vaccines against SARS-CoV-2 in patients with a history of anti-CD20 therapy (RituxiVac 2.0). Image Credit: NIAID
Study: Longitudinal analysis of antibody trajectories and humoral responses to a third dose of mRNA vaccines against SARS-CoV-2 in patients with a history of anti-CD20 therapy (RituxiVac 2.0). Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
The Study
A new study published on the medRxiv* preprint server analyzed the trajectories of anti-SARS-CoV-2 antibodies in patients from the RituxiVac study were compared to healthy volunteers. In addition, they investigated the immunogenicity of a third vaccine dose in previously non-responding patients.
The present study entailed a follow-up assessment of volunteers and patients from the RituxiVac Study. COVID-19-naive patients with a treatment history of anti-CD20 drugs with – rituximab or ocrelizumab and completion of a two-dose regimen of SARS-CoV-2 mRNA vaccination ≥4 weeks earlier were enrolled from April to June 2021.
The study included 33 patients and 26 healthy volunteers who had an initial humoral vaccination response and 32 patients who did not respond.
All participants initially received either the BNT162b2 mRNA COVID-19 vaccine (BioNTech/Pfizer) or the mRNA-1273 COVID-19 vaccine (Moderna).
Immunocompromised patients who were humoral non-responders after two vaccination doses were invited to receive a third dose of BNT162b2 mRNA Covid-19 vaccine or mRNA-1273 vaccine.
Participants were followed up as – initial non-responders, 4 weeks after the third vaccination dose, and initial responders – 6 months (± 2 months) after the second vaccination dose.
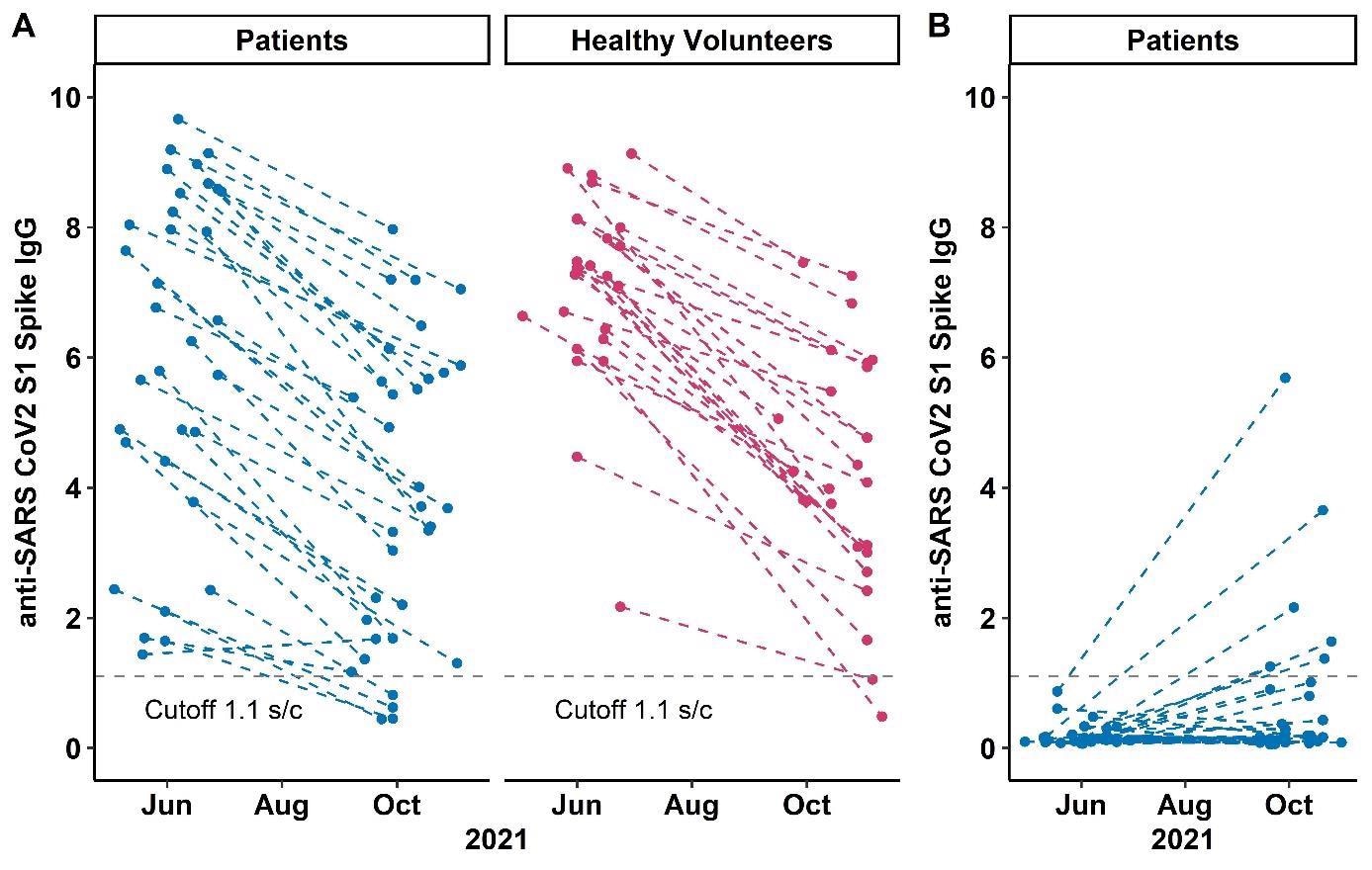 anti-SARS CoV2 S1 Spike IgG levels at study visits 1 and 2 in A) patients and volunteers with two-dose humoral response and B) patients with a third dose vaccination. Each point represents one study visit; intraindividual values are connected with dashed lines. The dotted grey line denotes the cut-off anti-SARS-CoV-2 S1-IgG value of 1.1 (signal to cutoff ratio).
anti-SARS CoV2 S1 Spike IgG levels at study visits 1 and 2 in A) patients and volunteers with two-dose humoral response and B) patients with a third dose vaccination. Each point represents one study visit; intraindividual values are connected with dashed lines. The dotted grey line denotes the cut-off anti-SARS-CoV-2 S1-IgG value of 1.1 (signal to cutoff ratio).
Findings
The findings depicted a comparable rate of decline in circulating anti-spike antibodies among patients on anti-CD20 therapies and healthy volunteers after a two-dose regimen of mRNA SARS-CoV-2 vaccines at the 6-month follow-up. Yet, 88% of the patients and 92% of volunteers had detectable antibody levels.
Immunocompetence parameters such as CD4, CD19, and total IgM levels, as well as rituximab treatment history, were not linked to antibody persistence. Only 19% of the subjects showed anti-S1 IgG seroconversion.
In terms of demographic, clinical, and immunocompetence indicators, three-dose responders were not significantly different from non-responders.
The persistence of circulating anti-spike antibodies was linked to higher initial antibody concentrations. It was reported that, after a third SARS-CoV-2 vaccination, only a small percentage of patients on anti-CD20 therapy who were two-dose non-responders acquired anti-spike antibodies. No clinical characteristics or immunocompetence biomarkers predicted humoral response following a third vaccine dose.
In inference, a subset of patients produces humoral immune response following the third dose of SARS-CoV-2 mRNA vaccines.
This result has also been supported by other recent reports where a comparable rate of roughly 25% de novo anti-spike seroconversion was found post third doses of SARS-CoV-2 vaccinations in patients (non-responders after a two-dose vaccination) on anti-CD20 therapy schedule.
When patients with a history of anti-CD20 therapy were compared to healthy volunteers, circulating antibodies decayed at comparable rates. and the initial titer magnitude is critical for the survival of anti-SARS-CoV2 antibodies over a 6-month timeframe. It was demonstrated that parameters like co-immunosuppression and circulating lymphocyte subpopulations could help determine immune responses to vaccination.
Future studies should take these aspects into account to facilitate tailored vaccination strategies and to establish the optimum time and amount of extra vaccine doses in the immunocompromised patients.
The present study does not consider an assessment of cell-mediated immunity, which might aid in understanding longer-lasting immune responses in anti-CD20 patients. Further, the report does not provide enough longitudinal observations to allow for in-depth modeling of antibody degradation dynamics, as proven in SARS-CoV-2 infection investigations or in selected reports of vaccinated healthy volunteers.
In a nutshell, the current investigation found comparable antibody reduction rates among patients with a history of CD20-depleting therapy and healthy volunteers but ineffective humoral responses to the third dose of SARS-CoV-2 mRNA vaccines in most two-dose non-responders. Thus, customized vaccination techniques for immunocompromised patients, stratified by B cell counts and first antibody titers, are required.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Sidler, D., Born, A., Schietzel, S., et al. (2021), “Longitudinal analysis of antibody trajectories and humoral responses to a third dose of mRNA vaccines against SARS-CoV-2 in patients with a history of anti-CD20 therapy (RituxiVac 2.0)”, medRxiv, doi: 10.1101/2021.11.19.21266572, https://www.medrxiv.org/content/10.1101/2021.11.19.21266572v1
- Peer reviewed and published scientific report.
Sidler, Daniel, Alexander Born, Simeon Schietzel, Michael S Horn, Daniel Aeberli, Jennifer Amsler, Burkhard Möller, et al. 2022. “Trajectories of Humoral and Cellular Immunity and Responses to a Third Dose of MRNA Vaccines against SARS-CoV-2 in Patients with a History of Anti-CD20 Therapy.” RMD Open 8 (1): e002166–66. https://doi.org/10.1136/rmdopen-2021-002166. https://rmdopen.bmj.com/content/8/1/e002166.