Researchers from Los Alamos National Laboratory in the United States developed a model to explain why severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections induce high inflammatory levels.
Their model focuses on cells that stimulate an inflammatory response by creating a feedback loop between infected and damaged cells. The persistent hyperinflammatory state makes it challenging to clear out damaged cells. Based on their results, antiviral treatment would have the most beneficial effect when administered early to prevent inflammation.
A preprint version of the study is available on the medRxiv* server while the article undergoes peer review.
 Study: A simple model of COVID-19 explains disease severity and the effect of treatments. Image Credit: NIAID
Study: A simple model of COVID-19 explains disease severity and the effect of treatments. Image Credit: NIAID

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Details of the proposed model
The researchers created a framework to explain the pathogenesis of severe coronavirus disease 2019 (COVID-19) illness and the mechanisms that incur symptoms such as high inflammation. The model focused on cells that stimulate inflammation through various patterns.
One group of cells trigger inflammation by being infected with SARS-CoV-2 and having pathogen-associated molecular patterns (PAMPs). This pattern may indicate hypoxia or a lack of nutrients for cells.
Another group is damaged cells that exhibit damage-associated molecular patterns (DAMPs). This may indicate the presence of pathogens.
They hypothesized that a feedback loop causes severe infection. High inflammatory levels triggered by SARS-CoV-2 cause extensive damage to uninfected cells and signal DAMPs that further stimulate the inflammatory response.
Findings
The model predicted that high inflammation levels would be a factor for severe infections. However, in a simulation of COVID-19 cases with hyper-inflammation, peak inflammation was not seen until later in disease progression.
The modeling results are similar to autopsy results of people who died from COVID-19 with hyperinflammation and high inflammatory biomarkers levels. The simulation prediction is that 13.5% of COVID-19 cases will have high inflammation, similar to the 14% observed in people infected with severe COVID-19 illness.
An analysis showed the underlying cause behind hyperinflammation. Results suggest that hyper-inflammation results from SARS-CoV-2 forcing the immune system into a constant state of high inflammation that does not clear out and continues way after the infection is gone.
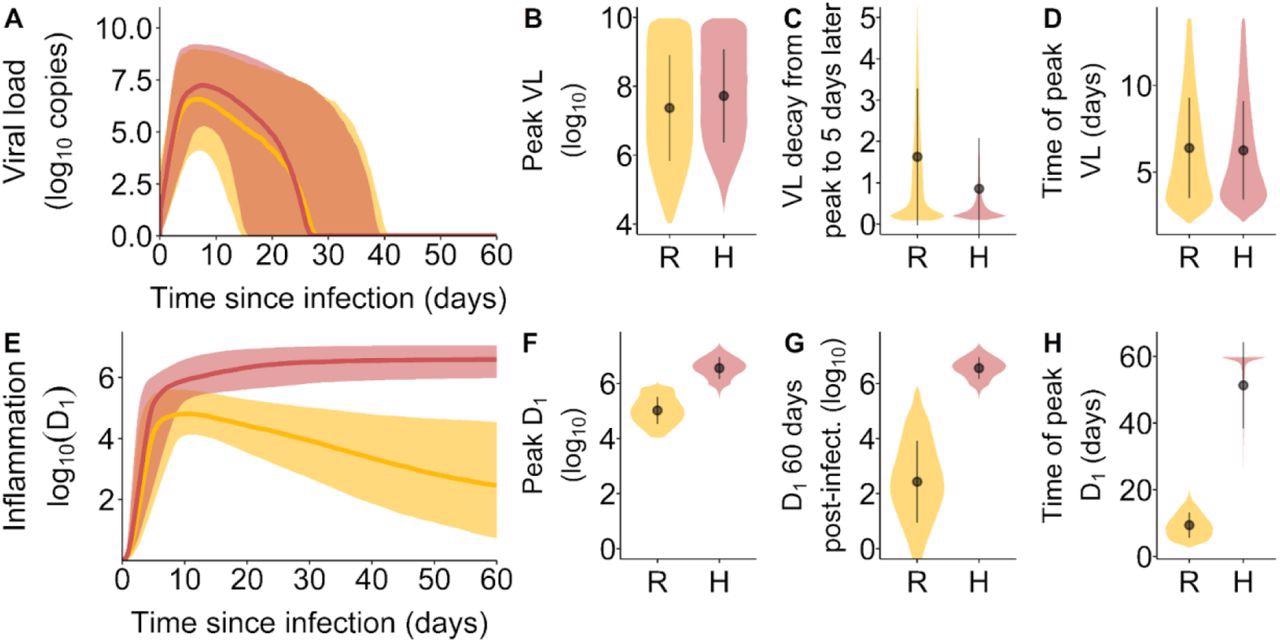 Viral load and inflammation trajectory characteristics by inflammation trajectory groups. (A) Viral loads over the course of infection. The shaded area corresponds to the 10th and 90th percentiles of the viral loads, while the curve represents the median. (B) The distribution of peak viral loads, (C) the VL decay from peak infection to 5 days after peak infection and (D) the time of occurrence of peak VL after infection. (E) Inflammation trajectories by inflammation trajectory groups. The shaded area corresponds to the 10th and 90th percentiles of D1, while the curve represents the median. (F) the distribution of peak D1, (G) the distribution of D1 at 60 days post-infection and (H) the time of occurrence of peak D1 after infection. In orange and represented by the symbol R, Resolved inflammation. In pink and represented by the symbol H, Hyperinflammation.
Viral load and inflammation trajectory characteristics by inflammation trajectory groups. (A) Viral loads over the course of infection. The shaded area corresponds to the 10th and 90th percentiles of the viral loads, while the curve represents the median. (B) The distribution of peak viral loads, (C) the VL decay from peak infection to 5 days after peak infection and (D) the time of occurrence of peak VL after infection. (E) Inflammation trajectories by inflammation trajectory groups. The shaded area corresponds to the 10th and 90th percentiles of D1, while the curve represents the median. (F) the distribution of peak D1, (G) the distribution of D1 at 60 days post-infection and (H) the time of occurrence of peak D1 after infection. In orange and represented by the symbol R, Resolved inflammation. In pink and represented by the symbol H, Hyperinflammation.
The amount of virus left in the body or viral load was also predictive of severe COVID-19 illness. Patients that shed the virus faster were more likely to have mild infections. Although, simulation results found that disease severity did not correlate with peak viral loads.
The model’s explanation between viral load and severe COVID-19 disease is because the body is taking a long time to clear out infected cells, increasing the risk for hyperinflammation. The model also showed that slow clearance prolonged PAMP and DAMP signaling leading to a persistent inflammatory response. Increased inflammation would most likely lead to a new set of damaged cells, further enhancing DAMP signaling.
Low viral clearance was one of the most important predictors for hyperinflammation.
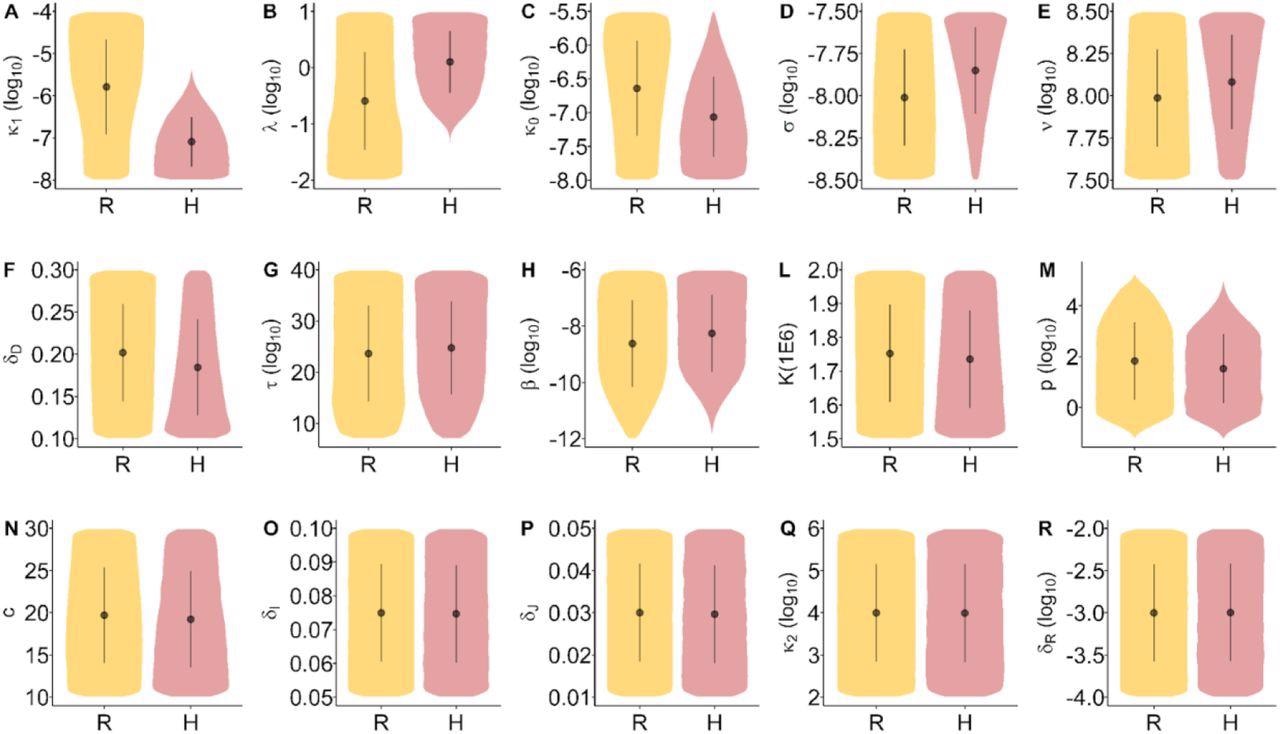 Distribution of the model parameters by inflammation trajectory groups. Distribution overlap may be discriminated from multivariate models. Inflammation trajectory groups R: Resolved inflammation, H: Hyperinflammation.
Distribution of the model parameters by inflammation trajectory groups. Distribution overlap may be discriminated from multivariate models. Inflammation trajectory groups R: Resolved inflammation, H: Hyperinflammation.
Prior research has suggested that severe COVID-19 is caused by an overactive immune response, particularly uncontrolled interferon (IFN) response. One function of type-I IFN is to produce an antiviral response in nonimmune cells.
Similarly, the research team’s current model shows that hyper-inflammation is associated with a weak IFN response. When type-I IFN response was strong, the model suggests there is a limit of cells with PAMPs and, as a result, a lower inflammatory state.
A weak interferon response may also prevent the activation of natural killer cells (NK cells). A delayed NK response could make it difficult to clear out cells who have had more time to emit PAMP and DAMP signaling. As a result, the body continues to experience high inflammation and disease severity.
The model also found that many innate immune cells were correlated with hyper-inflammation and severe illness.
Model simulations suggest that corticosteroids help lower bystander cell damage in patients with mild infection. But corticosteroids also make it harder for the body to clear out PAMP carrying cells, increasing disease severity.
The researchers suggest doctors administer antivirals early to prevent worsening disease severity and further inflammation.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources