Scientists have worked at an unprecedented speed to develop coronavirus disease 2019 (COVID-19) vaccines to contain the ongoing pandemic caused by the rapid spread of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). To date, several COVID-19 vaccines have received emergency use authorization (EUA) from global regulatory bodies, and vaccination programs have commenced in the majority of the countries of the world.
 Study: The T cell receptor repertoire reflects the dynamics of the immune response to vaccination. Image Credit: NIAID
Study: The T cell receptor repertoire reflects the dynamics of the immune response to vaccination. Image Credit: NIAID

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
How do Vaccines Protect Individuals from Diseases?
Vaccines offer prophylactic immunization against specific diseases by triggering a persistent, adaptive immune response that generates immunological memory, protecting against future infections. Typically, T cells offer adaptive immune protection, and the humoral antibody response is mediated by B cells. After vaccination, the B cells get activated and produce neutralizing antibodies that can bind to target proteins of a virus and, subsequently, protect the individual from the disease. The cell-mediated immune response is associated with the activation of the effector CD4 T cells, which stimulate B cells to produce antibodies. These cells may also employ other cells, such as macrophages, which possess microbicidal functions. Activated CD8 T cells eliminate virus-infected cells. Both CD4 and CD8 memory T cells persist long after vaccination and are primed to become effector T cells. Previous studies have shown that natural killer cells also possess memory-like behavior.
Through vaccines, pathogenic protein subunits are introduced in an individual that stimulates the B and T cells responses. Some of the common forms of vaccines are inactivated viruses, vectors that infect cells to generate viral proteins, messenger RNA (mRNA), and viral proteins. mRNA COVID-19 vaccines are likely to induce both CD4 and CD8 T cells responses.
Determination of Vaccine Effectiveness
In the current scenario, it is extremely important to measure the effectiveness of the vaccines as well as vaccination strategies. Typically, the response to vaccines is evaluated by detecting and measuring antibodies against viral proteins present in the serum. Vaccine response can also be evaluated by detecting activated B and/or T cells.
One of the shortcomings of antibody-based assays (e.g., ELISA) is that they cannot detect low levels of antibodies, which might be sufficient in protecting from a particular disease. In many instances, the levels of antibodies decrease significantly long after vaccination or natural infection, but they offer strong protection against the disease. Detectable levels of antibodies may develop, for antibody-based assays, at least a few weeks post-vaccination.
ELISpot is a technique that is used to detect T and B cells that are responsive to the vaccine. This assay can measure interferon-gamma or granzyme B from the activated cells. Although this method can indirectly assess the activity of immune cells, it is not always accurate.
Evaluation of Vaccine effectiveness Via T cell Receptors (TCR) Repertoire
A new study published on the bioRxiv* preprint server has hypothesized that T cell receptors (TCR) repertoire can measure the response to vaccines, as it would identify the TCR of clones that respond to the vaccination.
Previous studies have revealed that TCR is a heterodimer of two trans-membrane polypeptide chains (TCRα and TCRβ) linked by covalent disulfide bonds and a complete TCR repertoire can reflect the T cells present in an individual.
Researchers have developed a novel and accurate method known as Tseek, which is unbiased and provides sensitive profiling of TCRα and TCRβ chains or TCR repertoire by sequencing the TCR a and b chains. To evaluate the effectiveness of Tseek in the assessment of vaccine responses, researchers compared the responses to mRNA COVID-19 vaccines and the annual influenza vaccines. Based on epidemiological and antibody data, the vaccines have very different rates of efficacy, i.e., the effectiveness of the COVID-19 vaccine is 90%, whereas the influenza vaccine is 30%.
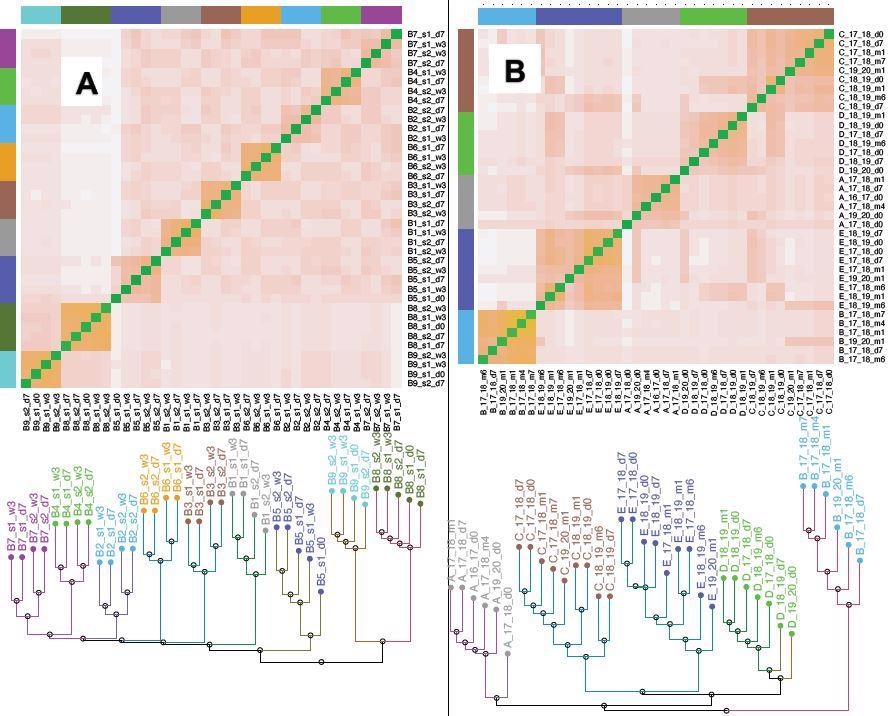 Clustering of T cell repertoire (CDR3 a) from the Covid/Flu vaccine samples. Distances between samples were defined for the CDR3 data using the Jensen-Shannon metric (an information-theoretic measure, Supplementary material). The heatmaps show the distance between pairs of samples, while the dendrograms show the samples cluster by an individual. A) Covid CDR3 b. The first dose is s1, the second dose is s2, d0 is the pre-vaccine sample, d7 is 7 days post-vaccination, w3 is 3 weeks post-vaccination. For each individual, the two samples after the second vaccine dose (s2_d7 and s2_w3), cluster separately from the two samples after the first vaccine dose (s1_d7 and s1_w3) suggesting distinct sets of T cells react to the two doses. The second dose broadens viral epitopes targeted, likely improving the immunity provided by the vaccination. (Fig. 3A shows the corresponding tree) B) Flu b CDR3 19_20 refers to the 2019-2020 flu season, d0 is the pre-vaccine sample, d7 is 7 days post-vaccination, m1 is 1-month post-vaccination. The clustering by vaccine dose does not occur in some individuals (B, D), and the m4 and m6 samples seem to have lost “memory” of the dose, the T cell response does not persist beyond 2-3 months. Even though there are responsive clones for each flu season (Table 2A, B), the relative lack of clustering of the tree by vaccine dose, in contrast to the covid vaccine data, suggests that the response is “weaker” and has more individual and seasonal variability. Fig. S3 shows data for the b.
Clustering of T cell repertoire (CDR3 a) from the Covid/Flu vaccine samples. Distances between samples were defined for the CDR3 data using the Jensen-Shannon metric (an information-theoretic measure, Supplementary material). The heatmaps show the distance between pairs of samples, while the dendrograms show the samples cluster by an individual. A) Covid CDR3 b. The first dose is s1, the second dose is s2, d0 is the pre-vaccine sample, d7 is 7 days post-vaccination, w3 is 3 weeks post-vaccination. For each individual, the two samples after the second vaccine dose (s2_d7 and s2_w3), cluster separately from the two samples after the first vaccine dose (s1_d7 and s1_w3) suggesting distinct sets of T cells react to the two doses. The second dose broadens viral epitopes targeted, likely improving the immunity provided by the vaccination. (Fig. 3A shows the corresponding tree) B) Flu b CDR3 19_20 refers to the 2019-2020 flu season, d0 is the pre-vaccine sample, d7 is 7 days post-vaccination, m1 is 1-month post-vaccination. The clustering by vaccine dose does not occur in some individuals (B, D), and the m4 and m6 samples seem to have lost “memory” of the dose, the T cell response does not persist beyond 2-3 months. Even though there are responsive clones for each flu season (Table 2A, B), the relative lack of clustering of the tree by vaccine dose, in contrast to the covid vaccine data, suggests that the response is “weaker” and has more individual and seasonal variability. Fig. S3 shows data for the b.
Scientists have evaluated the different outcomes of the vaccines using the Tseek method. In this context, they utilized PBMC samples from individuals who consistently received yearly influenza vaccines over several flu seasons for several years. Additionally, researchers collected PBMC samples from individuals who received two doses of COVID-19 mRNA vaccines were obtained. Neutralizing antibody titers were also measured in COVID-19 vaccine samples.
This study revealed that an individual’s TCR signature evolves gradually over the years in response to infections. Scientists found that SARS-CoV-2 vaccination induced a broad-spectrum T cell response that involved many expanded clones; however, this was not the case for the influenza vaccine, which elicited a narrower response involving fewer clones. In addition, many T cell clones provided temporal details that are typically not obtained from antibody measurements, especially, before the antibodies are detectable.
Conclusion
The current study revealed that TCR repertoire is a valuable biomarker for studying immune reactions to the vaccine. The authors highlighted some of the advantages of using Tseek to determine the response to vaccines, including it being a non-invasive approach, rapid assessment of vaccine efficacy, i.e., within a few days of vaccination, and a highly sensitive and specific method. The current study truly demonstrates that TCR repertoire sequencing can be effectively used for early and sensitive measurement of the adaptive immune response to vaccination, which can help improve the selection of the immunogen and optimize vaccine strategy.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.