The gold standard for detecting the presence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the reverse transcription-polymerase chain reaction (RT-PCR) assay that is conducted on respiratory specimens collected by healthcare professionals. Notably, droplet digital PC is gaining attention as a novel and highly sensitive testing assay; however, both methods require skilled technicians for sample collection by nasopharyngeal swab.
Background
Testing for SARS-CoV-2 by RT-PCR is a vital public health tool in the current coronavirus disease 2019 (COVID-19) pandemic. With the growing volume of tested samples, there is an increased demand for simple and self-collected samples for analysis.
Self-collected samples are increasingly being used as an alternative to nasopharyngeal swabs. Several studies have suggested their sensitivity as useful alternatives to nasopharyngeal swabs. However, there are limited data directly comparing several different types of self-collected materials to determine their usability.
In a recent Journal of Clinical Medicine study, researchers directly compare the diagnostic sensitivity of self-collected samples. They included saliva, tongue swab, nasal swab, chewed cotton pads, and gargle lavage with tap water samples and studied a large group of patients with known infection with SARS-CoV-2 in an unsupervised sample collection.
As secondary objectives, the authors of the current study also determined whether certain materials were more appropriate in certain age groups or in the presence of specific symptoms. They also investigated whether the duration since the last meal or tooth brushing had any influence on test sensitivity and surveyed the study participants on whether they experienced difficulty in collecting the samples.
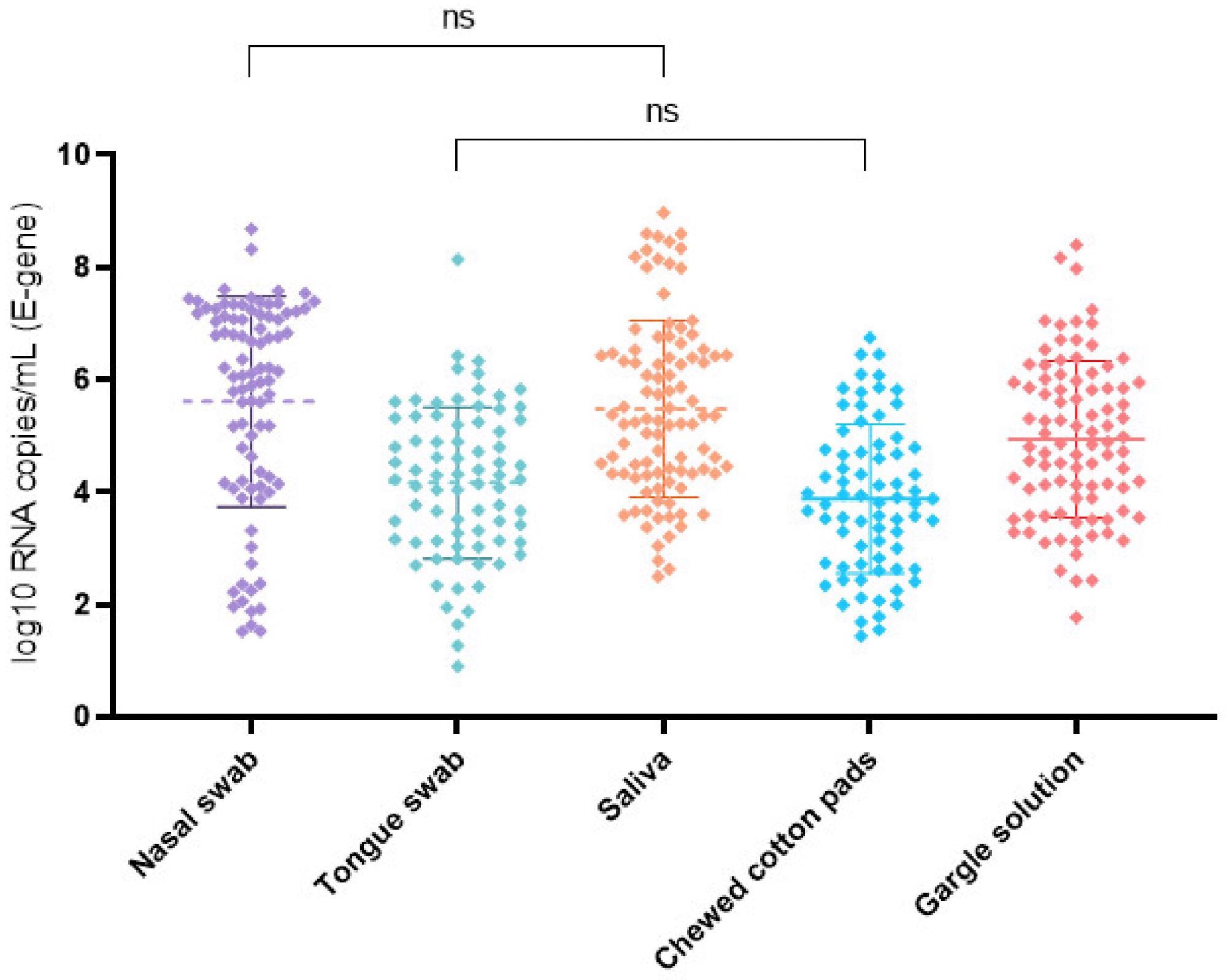 SARS-CoV-2 log10 RNA copies/mL for the E gene and examined self-collected specimens in order of collection including mean and standard deviation bars. The nasal swab and saliva had the highest mean viral load. The viral load was significantly lower (p < 0.05) in all other specimens. ns = no significant difference in the virus concentrations between the analyzed specimens.
SARS-CoV-2 log10 RNA copies/mL for the E gene and examined self-collected specimens in order of collection including mean and standard deviation bars. The nasal swab and saliva had the highest mean viral load. The viral load was significantly lower (p < 0.05) in all other specimens. ns = no significant difference in the virus concentrations between the analyzed specimens.
About the study
Individuals selected for the study were tested for SARS-CoV-2 at the Health Department of the City of Frankfurt in Germany or at the test center of the Kassenärztliche Vereinigung Frankfurt Messe. Samples were collected from consenting individuals between November 2020 to April 2021.
Inclusion criteria for the study were only applicable in the case of a positive RT-PCR in the professionally collected sample. Testing for SARS-CoV-2 was indicated for cases of recent contact with a person with COVID-19 or having symptoms of COVID-19.
A total of 102 predominantly symptomatic adults with a confirmed SARS-CoV-2 infection self-collected all forms of the aforementioned self-collected samples within 48 hours of initial diagnosis. Sample collection was unsupervised.
Study findings
Both native saliva and gargling with tap water had a high diagnostic sensitivity of 92.8% and 89.1%, respectively. Nasal swabs had a sensitivity of 85.1%, which was not significantly inferior to saliva; however, 16.6% of participants reported difficulty in self-collection of this sample. A tongue swab and saliva sample obtained by chewing a cotton pad had a significantly lower sensitivity of 74.2% and 70.2%, respectively.
The diagnostic sensitivity of any sample was not related to the presence of clinical symptoms or age. To this end, symptomatic patients appear to be capable of using self-collected specimens from saliva, gargle lavage, or mid-turbinate nasal swabs for diagnostic purposes. However, complementary experiments will be required to verify that differences in performance observed among the different sampling modes were not attributed to collection impairment.
In a direct comparison, both native saliva and gargling with tap water had high diagnostic sensitivity in the unsupervised sample collection process. Saliva sampling could detect SARS-CoV-2 positive cases in 92.8% of study participants, whereas gargling with plain tap water identified 89.1%.
This adds to evidence from previous studies that have shown that native saliva is useful to test for the presence of SARS-CoV-2 by RT-PCR with fair or good agreement to the nasopharyngeal swab samples in both symptomatic and asymptomatic patients.
Implementation
The use of self-collected samples without expert supervision or skilled technicians can be helpful in testing strategies. Owing to the ease of the ample-collecting processes, these sample collection procedures can also help improve patient adherence. However, one should prefer native saliva, gargling water, or mid-turbinate nasal swabs over tongue samples for better results.
The availability of self-collected samples is especially important for repeated testing in hazardous environments. Further studies assessing the effect of sample collection impairment and their effect on the sensitivity of each self-collection method should be conducted.
Journal reference:
- Kohmer, N., Eckermann, L., Böddinghaus, B., et al. (2021). Self-Collected Samples to Detect SARS-CoV-2: Direct Comparison of Saliva, Tongue Swab, Nasal Swab, Chewed Cotton Pads and Gargle Lavage. Journal of Clinical Medicine 10(5751). doi:10.3390/jcm10245751, https://www.mdpi.com/2077-0383/10/24/5751