
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The current vaccination programs have effectively reduced severe outcomes of coronavirus disease 2019 (COVID-19). However, the immunity elicited by COVID-19 vaccines is believed to wane with time combined with the emergence of new variants of SARS-CoV-2 has threatened the protection provided by vaccines, especially in vulnerable populations such as the elderly.
A better understanding of the correlation between nAb and different variants of SARS-CoV-2 will help in knowing whether the increase in breakthrough infections, hospitalization, and deaths are due to waning immunity or new variants.
About the study
The present study utilized data from the CONSENSUS study, from which 37 elderly individuals between 70 and 89 years old who had received two doses of the BNT162b2 vaccine at about three weeks apart were selected. The researchers determined the correlation between the SARS-CoV-2 nAb and different variants of SARS-CoV-2 through enzyme-linked immunoassay (ELISA), receptor-binding domain (RBD) ELISA, and nAb titers with D614G pseudotype as the control sample.
Based on the age factor, the researchers classified the study subjects into two cohorts including those between the ages of 70-79 years (n= 24) and 80-89 (n=13) years. Using a SARS-CoV-2 spike pseudotype-based micro-virus neutralization assay (mVNT), the neutralization titer (ND80) was determined in the serum samples of both the cohorts at 3-weeks and 20-weeks post-second dose.
Study findings
At three weeks post-vaccination, the median titer for 70-79 years and 80-89 years cohort was 128.1 and 62.6, respectively, which later dropped to 24.84 and 16.0 at 20-weeks post-vaccination. Among the 70-79-year-old cohort, 12.5% of the subjects had no detectable nAbs, whereas 23.1% in the 80-89-year-old cohort had no detectable nAbs at three weeks post-vaccination.
At 20-weeks post-vaccination the number of subjects who had no detectable nAbs increased to 18.2% and 30.8% in the 70-79-year-old and 80-89-year-old cohorts, respectively. Overall, the nAb titers dropped 4.9-fold between three weeks and 20 weeks post-vaccination, with 21.6% of individuals having no detectable nAbs.
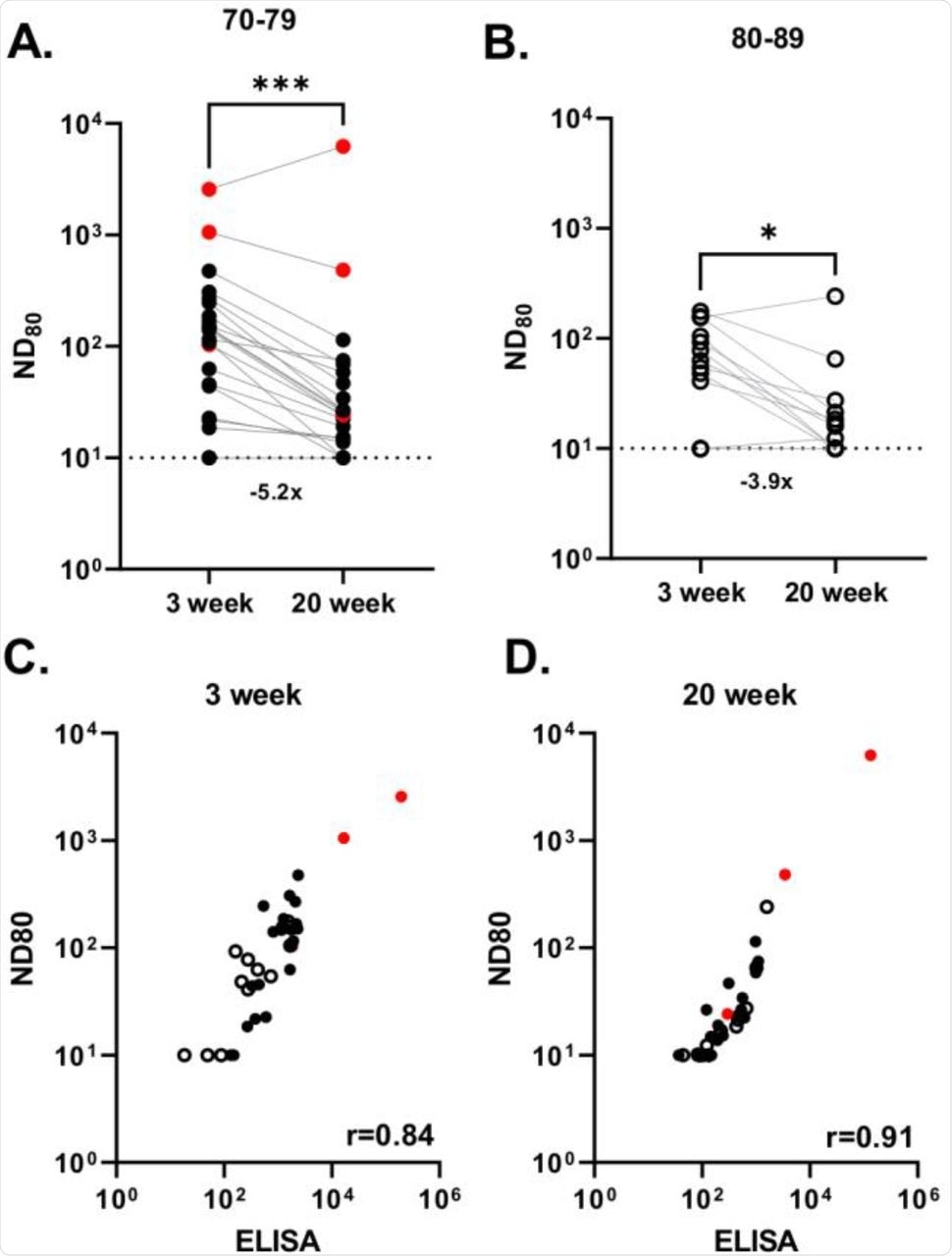
Neutralizing antibody responses generated following BNT162b2 vaccination.
The researchers investigated the neutralization of SARS-CoV-2 variants of concern (VOC); Alpha (B.1.1.7), Beta (B.1.351), and Delta (B.1.617.2), as well as the D614G-containing lineage B.1. As compared to D614G, the 70-79-year-old cohort showed 1.1, 33.3, and 9.0 fold reduction in the neutralization of Alpha, Beta, and Delta antibodies, respectively. Among 80-89 years of age, reduction in nAbs was 136.6, 10, and 12.4 for Alpha, Beta, and Delta variants, respectively.
The researchers then performed targeted ELISA with recombinant RBDs reflecting wild-type (WT) SARS-CoV-2, Alpha, Beta, and Delta sequences. To this end, no significant difference in ELISA titers between Alpha and Delta RBDs in both the cohort groups was observed; however, the Beta RBD showed a significant reduction in binding as compared to the WT.
The VOC Beta showed a weak correlation between RBD-ELISA and nAbs titers. Comparatively, the correlation for WT, Alpha, and Delta was relatively consistent.
The examination of the nAbs for 21 different SARS-CoV-2 variant spikes from the same cohort sample pool at three weeks post-vaccination confirmed a significant potential for antigenic escape especially for the Omicron (BA.1), Beta (B.1.351), Delta (B.1.617.2), Theta 27 (P.3), C.1.2, and B.1.638 variants. Surprisingly, the median titer of Delta was lower than the recently emerged sub-lineage AY.4.2.
The researchers extended their study to identify single amino acid changes at positions K417, L452, T478, or E484 through RBD-ELISAs. To this end, they found K417N (1.2-fold), E484K (1.6-fold), and E484D (1.4-fold) mutations responsible for the significant drop in neutralization, which indicated the importance of these positions for escaping neutralization.
For subjects of the CONSENSUS trial who had received a third booster dose, the researchers performed mVNT assays on serum samples collected at four weeks post booster dose and compared with previous assays with post-second-dose vaccination. An estimated 83% and 86% of participants in the 70-79-year-old and 80-89-year-old cohort, respectively, showed no detectable nAbs at three weeks post the second dose of vaccination.
Comparatively, 92% and 86% of subjects showed no detectable nAbs at 20 weeks post the second dose, respectively. The samples collected at four weeks post booster vaccine dose showed significant detectable titers against the SARS-CoV-2 Omicron variant. The correlation of S ELISA and Omicron nAb titer showed improved neutralization titers after the third dose of vaccination.
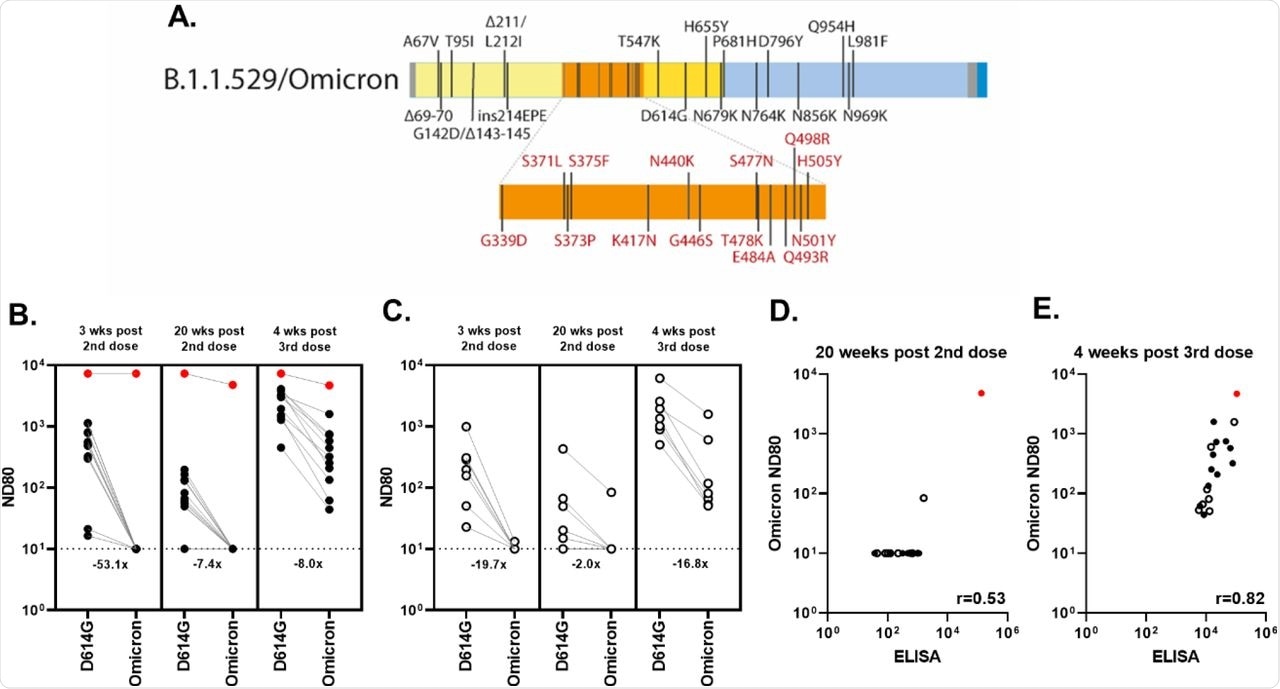
Neutralizing antibody responses to Omicron after a 3rd booster dose of BNT162b2.
Overall, the findings of the present study indicate that SARS-CoV-2 nAbs wane over time. Due to the emergence of new SARS-CoV-2 variants, information on the antigenicity of these new trains and their interaction with vaccine-induced Abs may be essential in developing appropriate vaccination strategies to protect the most vulnerable groups in society.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Newman, J., Thakur, N., Peacock, T. P., et al. (2021). Neutralising antibody activity against SARS-CoV-2 variants, including Omicron, in an elderly cohort vaccinated with BNT162b2. medRxiv. doi:10.1101/2021.12.23.21268293. https://www.medrxiv.org/content/10.1101/2021.12.23.21268293v1
- Peer reviewed and published scientific report.
Newman, Joseph, Nazia Thakur, Thomas P. Peacock, Dagmara Bialy, Ahmed M. E. Elrefaey, Carlijn Bogaardt, Daniel L. Horton, et al. 2022. “Neutralizing Antibody Activity against 21 SARS-CoV-2 Variants in Older Adults Vaccinated with BNT162b2.” Nature Microbiology 7 (8): 1180–88. https://doi.org/10.1038/s41564-022-01163-3. https://www.nature.com/articles/s41564-022-01163-3.