New kidney research from the University of Virginia School of Medicine is raising concerns that long-term use of drugs commonly prescribed to treat high-blood pressure and heart failure could be contributing to kidney damage.
Patients should continue taking the medications, which include the well-known and widely used ACE inhibitors, the researchers say. But the scientists are urging studies to better understand the drugs' long-term effects.
Our studies show that renin-producing cells are responsible for the damage. We are now focusing on understanding how these cells, which are so important to defend us from drops in blood pressure and maintain our well-being, undergo such transformation and induce kidney damage. What is needed is to identify what substances these cells make that lead to uncontrolled vessel growth."
Maria Luisa Sequeira Lopez MD, UVA's Department of Pediatrics and Child Health Research Center
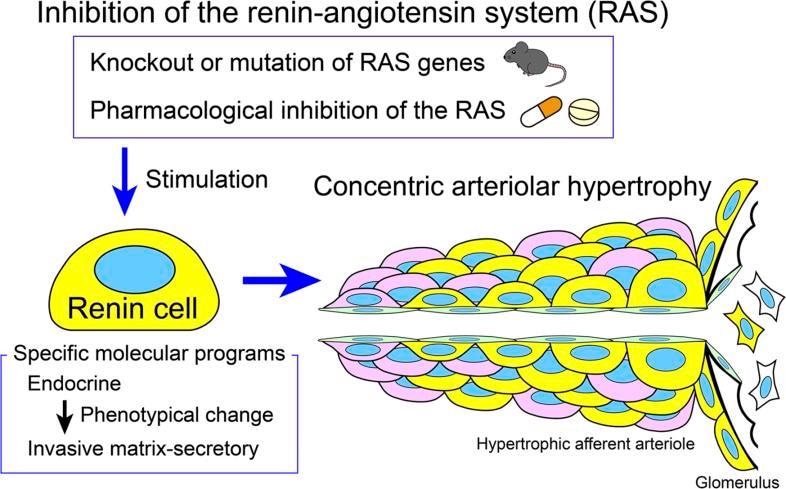
The causes of kidney damage
Chronic high blood pressure affects a billion people around the world. The UVA researchers wanted to better understand why severe forms of the condition are often accompanied by thickening of the arteries and small blood vessels in the kidney, leading to organ damage.
They found that specialized kidney cells called renin cells play an important role. These cells normally produce renin, a vital hormone that helps the body regulate blood pressure. But harmful changes in the renin cells can cause the cells to invade the walls of the kidney's blood vessels. The renin cells then trigger a buildup of another cell type, smooth muscle cells, that cause the vessels to thicken and stiffen. The result: Blood can't flow through the kidney as it should.
Further, the researchers found, long-term use of drugs that inhibit the renin-angiotensin system, such as ACE inhibitors, or angiotensin receptor blockers, have a similar effect. These drugs are widely used for many purposes, including treating high blood pressure, congestive heart failure and heart attacks, as well as to prevent major heart problems. But long-term use of the drugs was associated with hardened kidney vessels in both lab mice and humans, the scientists found.
The researchers note that the medications can be lifesaving for patients, so they stress the importance of continuing to take them. But they say additional studies are needed to better understand the drugs' long-term effects on the kidneys.
"It would be important to conduct prospective, randomized controlled studies to determine the extent of functional and tissue damage in patients taking medications for blood pressure control," said Ariel Gomez, MD, of UVA's Department of Pediatrics and Child Health Research Center. "It is imperative to find out what molecules these cells make so that we can counteract them to prevent the damage while the hypertension is treated with the current drugs available today."
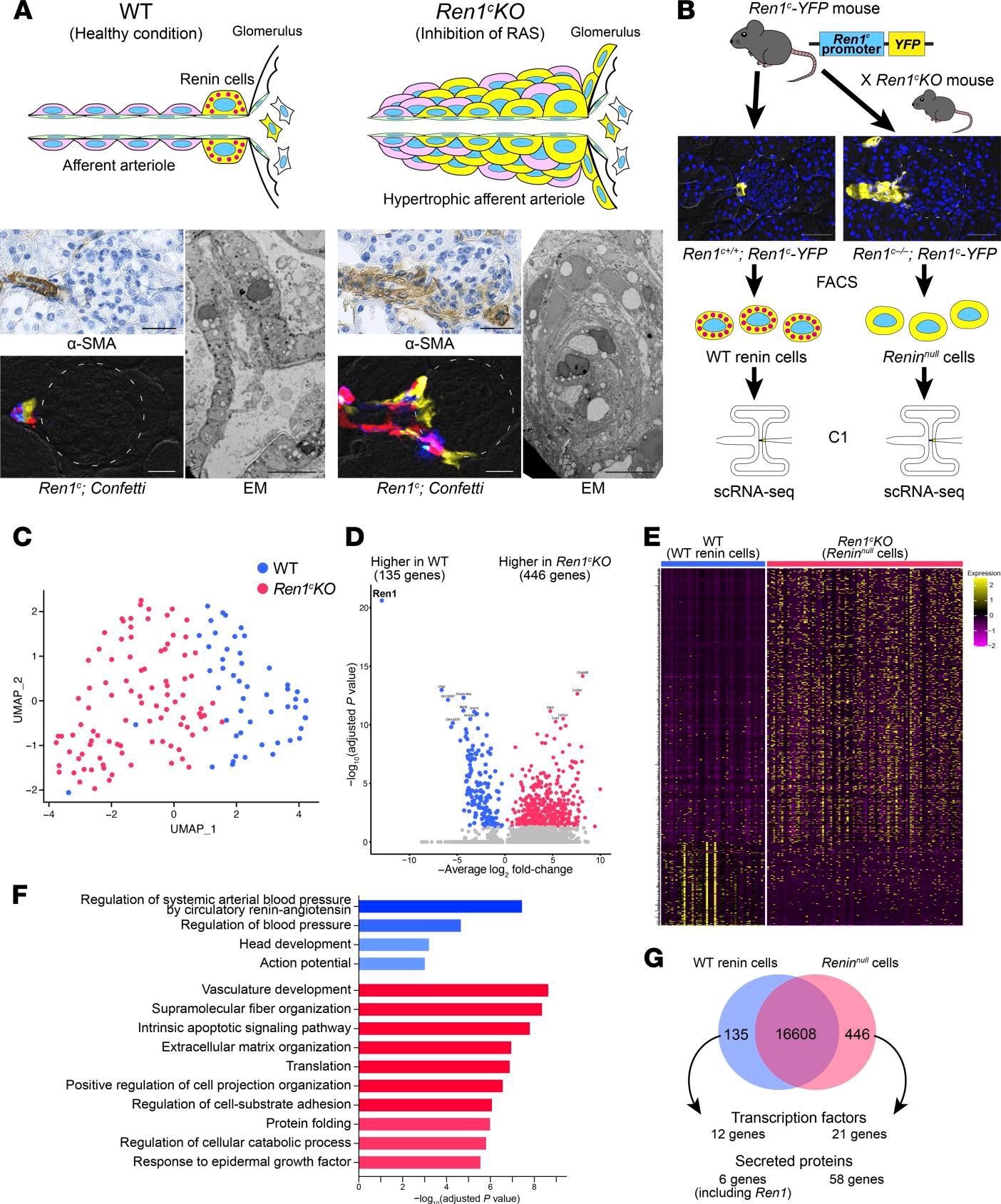
Reninnull cells have different transcriptomic profiles. (A) Pathological findings of Ren1c-KO mice. Immunohistochemistry for α-SMA showed hypertrophic arterioles in Ren1c-KO mouse kidneys. Scale bars: 20 μm. Kidney sections from Ren1c-KO Ren1c-Cre Confetti mice showed multiple colors in the hypertrophic arterioles, suggesting the multiclonality of Reninnull cells. Scale bars: 20 μm. Left: WT mice showed normal architecture of periglomerular arterioles with a single layer of smooth muscle cells (SMCs). Right: Ren1c-KO mice showed concentric hypertrophy of arteriolar smooth muscle. Scale bars: 10 μm. (B) Schematic of scRNA-Seq of WT renin cells and Reninnull cells. Ren1c-YFP mice have a transgene consisting of the YFP genes driven by the Ren1c gene regulatory region. YFP-positive kidney cells from Ren1c+/+ Ren1c-YFP mice and Ren1c–/– Ren1c-YFP mice were isolated by FACS. Single cells were captured with the Fluidigm C1 System, and scRNA-Seq was performed. Scale bars: 50 μm. (C) The UMAP with all the cells after normalization. (D) Volcano plot showing the differentially expressed genes between WT renin cells and Reninnull cells. (E) Heatmap analysis with differentially expressed genes showed a clear separation of WT renin cells and Reninnull cells. (F) The 10 most enriched categories identified by GO analysis on genes higher in WT renin cells (red) and genes higher in Reninnull cells (blue). (G) Venn diagram showing differentially expressed genes. α-SMA, α–smooth muscle actin; EM, electron microscopy; FACS, fluorescence-activated cell sorting; GO, Gene Ontology; Ren1c-KO, Ren1c gene knockout; scRNA-Seq, single-cell RNA sequencing; UMAP, uniform manifold approximation and projection; WT, wild-type.
Findings published
The researchers have published their findings in the scientific journal JCI Insight. The article was selected as a cover story. The research team consisted of Hirofumi Watanabe, Alexandre G. Martini, Evan A. Brown, Xiuyin Liang, Silvia Medrano, Shin Goto, Ichiei Narita, Lois J. Arend, Sequeira-Lopez and Gomez.
The research was supported by the National Institutes of Health, grants P50 DK 096373, R01 DK 116718, R01 DK 116196, R01 DK 096373 and R01 HL 148044; and the Japan Society for the Promotion of Science Overseas Research Fellowships.
Source:
Journal reference:
Watanabe, H., et al. (2021) Inhibition of the renin-angiotensin system causes concentric hypertrophy of renal arterioles in mice and humans. JCI Insight. doi.org/10.1172/jci.insight.154337, https://insight.jci.org/articles/view/154337