Over the past several years, considerable effort has been made towards developing better diagnostic and prognostic tools. However, until now, the focus of research has mainly been on the virus itself or the immune response it triggers. To unify diagnostic and prognostic tools for COVID-19 analyses, researchers propose using extracellular vesicles (EVs) from serum liquid biopsies.
EVs play a crucial role in intercellular signaling, primarily as they affect immune responses. In their study, the team demonstrates a novel signature of SARS-CoV-2 infection using innate and adaptive immune EVs profiling, together with SARS-CoV-2 Spike S1+ EVs.
Additionally, they provide a unique tool for monitoring the severity of disease progression by tracing the coexistence of viral and host cell signatures.
The study “Serum extracellular vesicles trace COVID-19 progression and immune responses” was recently posted to the medRxiv* preprint server.
Study: Serum extracellular vesicles trace COVID-19 progression and immune responses. Image Credit: Fit Ztudio / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
How they did it
To study extracellular vesicles, the researchers analyzed serum samples from 20 patients with mild COVID-19 infection and 17 people with no history of SARS-CoV-2 infection. All patients with COVID-19 disease had mild symptoms, including fever, fatigue, and muscle weakness.
A nanoparticle analyzer was used to look at the size and concentration of serum extracellular vesicles subsets. Results showed that patients with COVID-19 infection had an abundance of small extracellular vesicles and CD66b+ extracellular vesicles compared to healthy donors.
The COVID-19 infection group also showed a more significant reduction in extra-large CD63+, CD38+, IgA+, and IgG+ extracellular vesicles than the control group.
Depletion of extracellular vesicles in the early onset of COVID-19 disease
Patients with COVID-19 illness have dysregulated immune cells functioning, but whether it’s because of COVID-induced changes in extracellular vesicles remains unknown.
The research team first looked at the canonical leukocyte marker CD45 in serum samples. Serum samples from mild cases had decreased the number of CD45+ extracellular vesicles. They also found a significant reduction in CD45+ extracellular vesicles 3 to 13 days after showing symptoms.
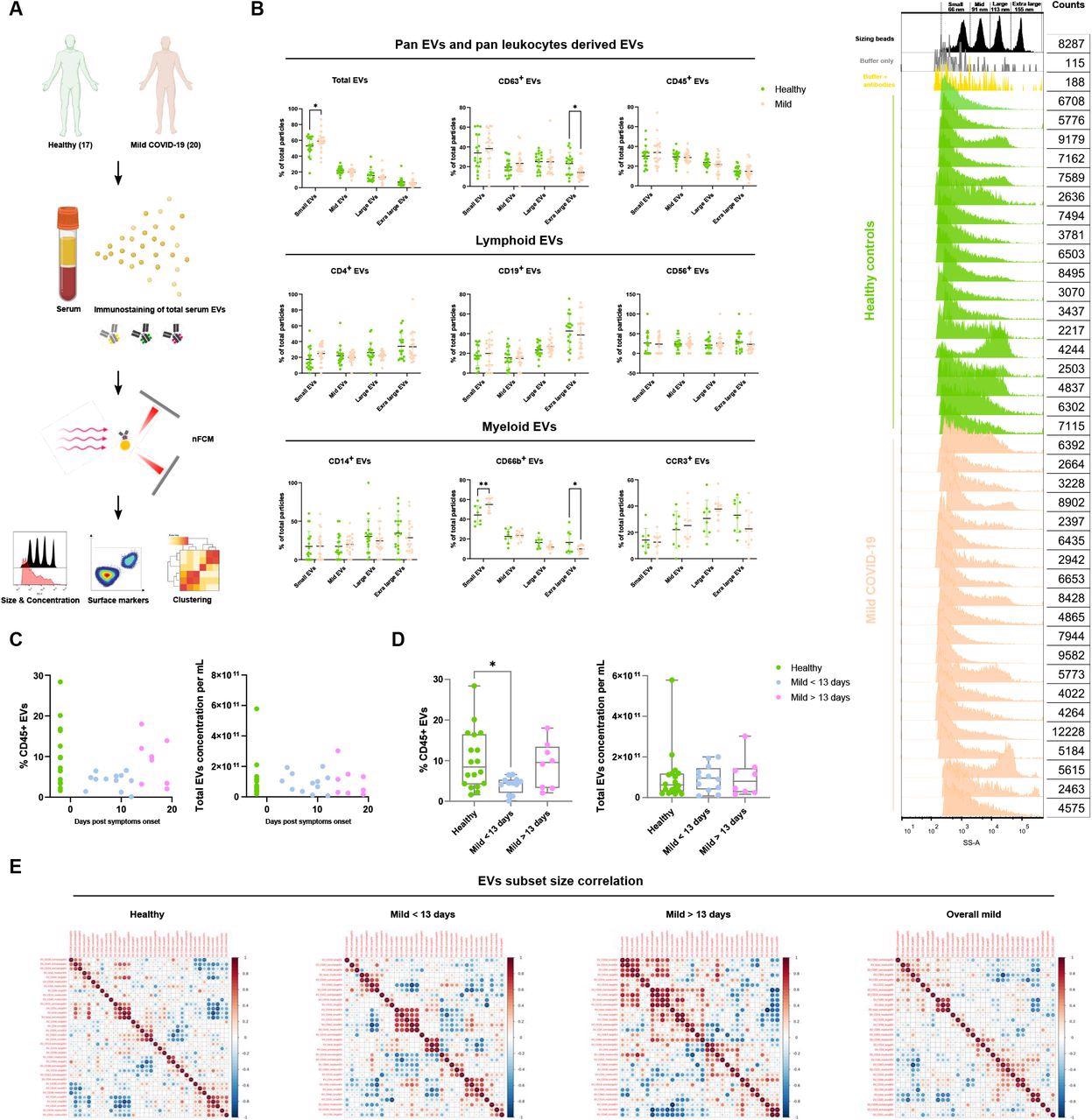
Characterization of immune serum EVs in healthy controls and mild COVID-19 patients. (A) Schematic outline of EVs profiling from denoted human samples. (B) Size distribution quantification of serum EVs from denoted human samples and different EV subsets, with size reference beads with a mixture of four modal sizes of 66 nm (small), 91 nm (medium), 113 nm (large), 155 nm (extra-large). Representative side scatter histogram of size reference beads in (B) and total serum EVs from denoted human samples on the right. (C, D) Quantification of total serum EVs and CD45+ EVs in denoted human samples at days of reported symptom onset. (E) Spearman’s rank correlation matrix of size distribution of serum EVs subsets between healthy donors and mild COVID-19 patients. One-way ANOVA, p < 0.05 *, p < 0.01 **, p < 0.005 ***.
Interestingly, the researchers found CD45+ extracellular vesicle levels restored in infected patients 13 days after symptom onset.
A significant correlation between large and extra-large CD31+ extracellular vesicles with the total concentration of extracellular vesicles was found in healthy donors and serum samples of patients who had symptoms for 13 days.
Serum samples from infected patients who showed symptoms for less than 13 days strongly correlated with small CD14+, CD19+, CD56+, and CD63+ extracellular vesicles.
“These data suggest that classical monocytes-, B 118 cells- and natural killer cells-derived small EVs are predominantly affected in the early stage of COVID-19,” said the researchers.
SARS-CoV-2 manipulate extracellular vesicles to trick antibodies and increase disease progression
Prior research has suggested that SARS-CoV-2 Spike S1 containing extracellular vesicles could serve as decoys to fool neutralizing antibodies. The researchers tested this theory by looking for Spike S1+ extracellular vesicles in serum samples of infected patients.
The team detected spike S1+ extracellular vesicles in healthy serum samples, which the researchers explain might be from cross-reactive epitopes that bind to spike S1 antibodies. In the COVID-19 group, five out 16 patients had over 1.75% of spike S1 serum extracellular vesicles.
Immunostaining showed that serum samples collected from patients who had symptoms for less than 13 days had significantly higher amounts of spike S1+CD31+ extracellular vesicle levels in the endothelial tissues than healthy serum samples and serum samples of patients with symptoms for over 13 days.
The results confirm the existence of SARS-CoV-2 specific extracellular vesicles in infected patients and likely originate in SARS-CoV-2 infected endothelial tissues.
Predicting disease severity with extracellular vesicles
An exploratory analysis showed extracellular vesicle levels correlated with disease severity. Healthy donors showed a high association with CD45+ extracellular vesicles. Moreover, CD38+ extracellular vesicles were significantly associated with high levels of IgG+ extracellular vesicles.
Patients with symptoms for less than 13 days had high levels of Spike S1+ extracellular vesicles. High amounts of S1+ containing and spike S1+ CD31+ extracellular vesicles were negatively correlated with CD19+ and CD66b+ extracellular vesicles.
Serum samples from patients with symptoms for more than 13 days were associated with high CD31+ and CD63+ extracellular vesicles.
The researchers conclude, “the dynamics of serum EVs subsets distribution highlighted their predictive values in the perspectives of overall host immune responses and correlation between disease-specific Spike S1+ EVs and immune responses during COVID-19 progression. The study strengthens the potential of serum EVs based diagnostic and prognostic, potentially therapeutic applications in COVID-19, and easily transferred to other types of viral infections and cancers.”

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources