
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Introduction
Globally, more than 358 million individuals have been infected by SARS-CoV-2 since its emergence in Wuhan, China, in late December 2019. Since the pandemic's beginning, the possibility of transmission of SARS-CoV-2 from humans to animals has been widely discussed, although the emphasis was placed on identifying susceptible species and potential intermediate or reservoir hosts.
About the study
In the present study, researchers conducted serological screenings of 1,000 samples from cattle kept in 83 holdings located in the German states of Bavaria, Lower Saxony, Saxony-Anhalt, and Thuringia. These samples were collected between autumn 2021 and early winter 2022, when the wave of infections in humans caused by the SARS-CoV-2 Delta variant of concern (VOC) was on the rise. From each of the 83 holdings, two to 20 serum or plasma samples were collected and analyzed.
The analysis was performed using a receptor-binding domain (RBD)-based multispecies enzyme-linked immunosorbent assay (ELISA). The ELISA results were further confirmed by an indirect immunofluorescence assay (iIFA) using SARS-CoV-2-infected Vero cells. These results were then confirmed using a surrogate virus neutralization test (sVNT) which mimicked the relationship between SARS-CoV-2 and the host cell's membrane receptor protein angiotensin-converting enzyme-2 (ACE-2) to detect the presence of neutralizing antibodies. Although highly specific, sVNT was found to be only moderately sensitive for bovine samples.
Results
The results showed that 11 cattle from nine holdings tested positive for COVID-19 using the RBD-ELISA. Among these 11 cattle, 10 RBD-ELISA results were confirmed by indirect immunofluorescence assay (IIFA). A titer range between 1/8 and 1/512 was observed in seropositive cattle by IIFA. Four out of the 11 samples were further tested and confirmed to be positive by the sVNT.
The study confirmed that ELISA does not react with the bovine coronavirus (BCoV) and found no correlation between BCoV infection and SARS-CoV-2 infection, indicating the possibility of double infections in cattle.
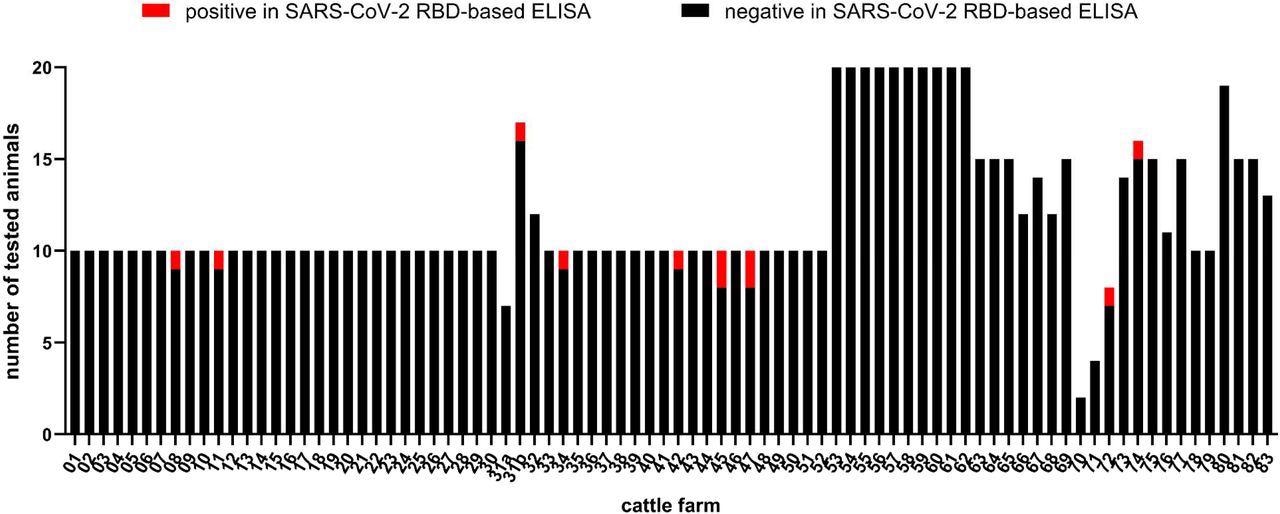
Number of cattle per farm tested for antibodies against SARS-CoV-2. Samples that reacted negative in the RBD-based ELISA are depicted in black and positive samples in red. Holding 31 was sampled twice (indicated as 31a and 31b), in between the animal owner was quarantined.
Conclusion
Based on the study findings, the researchers conclude that occasional spill-over of SARS-CoV-2 infections from humans to cattle is possible. However, intraspecies transmission is found to be rare in the holdings studied. The authors believe that future SARS-CoV-2- transmission monitoring programs should include cattle farms to surveil the spread of the virus. In particular, the spread of BCoV, a coronavirus highly prevalent in the cattle population, is of significant concern.
Double infections of SARS-CoV-2 and BCoV could lead to recombination between both viruses, creating a new virus. While an emergence of this kind is unlikely due to the low SARS-CoV-2-susceptibility in cattle, the likelihood can still be controlled by appropriate investigations and screening to exclude the transmission of novel variants in livestock.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Serological screening suggests single SARS-CoV-2 spillover events to cattle, Kerstin Wernike, Jens Boettcher, Silke Amelung, Kerstin Albrecht, Tanja Gaertner, Karsten Donat, Martin Beer, bioRxiv 2022.01.17.476608, doi: https://doi.org/10.1101/2022.01.17.476608, https://www.biorxiv.org/content/10.1101/2022.01.17.476608v1
- Peer reviewed and published scientific report.
Wernike, Kerstin, Jens Böttcher, Silke Amelung, Kerstin Albrecht, Tanja Gärtner, Karsten Donat, and Martin Beer. n.d. “Antibodies against SARS-CoV-2 Suggestive of Single Events of Spillover to Cattle, Germany - Volume 28, Number 9—September 2022 - Emerging Infectious Diseases Journal - CDC.” Wwwnc.cdc.gov. Accessed May 10, 2023. https://doi.org/10.3201/eid2809.220125. https://wwwnc.cdc.gov/eid/article/28/9/22-0125_article.