When a drug molecule binds to its target protein, it stays bound for some time before eventually unbinding the target. The actual time how long a drug molecule resides bound to its target varies among compounds. The lifetime of the drug-target complex may play a crucial role in drug efficacy, as a long target residence time can, in some cases, be important for drug efficacy. Therefore, understanding its underlying causes enables more rational drug design.
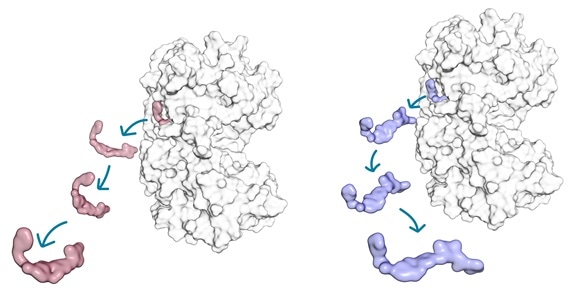
In the study, researchers set out to resolve why two structurally similar small molecule kinase inhibitors (red and blue) exhibit different lifetime of the drug-target complex with their target protein kinase (white). Photographer: Tatu Pantsar
In the new study, researchers from the University of Eastern Finland and the University of Tübingen identified the key elements driving for a long or a short target residence time among similar small molecule kinase inhibitors on the atomistic level. The findings were published in Nature Communications.
Dozens of small molecule kinase inhibitors have already been approved for clinical use, most of them for the treatment of cancer.
“Initially, we were interested in what causes the discrepancy in the target residence time between two similar small molecule kinase inhibitors,” says Senior Researcher, lead author Tatu Pantsar from the University of Eastern Finland.
Prof. Stefan Laufer’s group at the University of Tübingen has designed, synthesized and biologically characterized numerous small molecule kinase inhibitors for a protein kinase called p38a MAPK, which enabled this research.
In the study, we focused on two small molecule kinase inhibitors that are equipotent in isolated enzyme assays but show difference in their target residence times, i.e., for how long a single small molecule kinase inhibitor is bound to the target protein. We also found that the inhibitor with a longer target residence time was more potent when tested in cells,”
Tatu Pantsar, Senior Researcher, Lead Author, University of Eastern Finland.
In the study, researchers studied and compared the small molecule kinase inhibitors together with their target protein using computer simulations that were conducted on Finnish supercomputers.
The protein behaves differently depending on the bound inhibitor.
“The simulations suggest that when a small molecule inhibitor is bound to the protein, the protein is more dynamic when the short residence time inhibitor is bound to it. This basically means that the protein moves more when it is binding the short residence time inhibitor, and less when it is binding the long residence time inhibitor,” Pantsar points out.
Water molecules have a great impact on the drug target residence time.
“These tiny yet abundant water molecules surrounding the protein appear to be really important. When the inhibitor unbinds the protein, water molecules will take their place in the binding site.”
In the simulations, the long residence time inhibitor was less exposed to the water molecules, and the required energy for the water molecules to reoccupy the binding site of the long residence time inhibitor was much higher. This results in a higher energetical barrier for the inhibitor to dissociate from its target and thereby in a longer lifetime of the drug-target complex.
The observations on target protein behaviour and the role of water molecules were also confirmed with a structurally diverse small molecule kinase inhibitor that has an extremely short residence time.
The results can be useful in the early stages of drug design.
“Now that we understand better the atomistic level reasons that define the drug residence time, it enables more rational small molecule design that can be applied in a drug discovery project if a long target residence time is desired. Obviously, one needs to remember that the target residence time is just one aspect in the very complex and difficult drug design process, where a multitude of different things need to be considered,” Pantsar concludes.
At the University of Eastern Finland, the research was carried out at the School of Pharmacy and within the DrugTech Research Community. The research was made possible by the computational resources provided by CSC – IT Center for Science Finland.
Source:
Journal reference:
Pantsar, T., et al. (2022) Decisive role of water and protein dynamics in residence time of p38α MAP kinase inhibitors. Nature Communications. doi.org/10.1038/s41467-022-28164-4.