Human testis cells express angiotensin-converting enzyme 2 (ACE2) receptors, which mediate the SARS-CoV-2 entry to host cells. Several studies have suggested that men are more affected by SARS-CoV-2 infection than women; it is thus crucial to study SARS-CoV-2 tropism in testis and evaluate the impact of SARS-CoV-2 infection on male fertility.
While previous studies have demonstrated testicular alterations promoted by SARS-CoV-2 infection, in-depth testicular pathogenesis, including the cellular, enzymatic, hormonal, and critical genetic alterations in the testes of COVID-19 patients, remains unclear.
About the study
In the present study, researchers collected the testicles of 11 non-vaccinated male patients deceased from COVID-19 complications. They collected testicles through an incision on the median raphe of the scrotum within three hours of the patient's death. They incised fragments of testicular parenchyma and stored them in RNAlater® solution to perform viral and testicular genetic studies later.
For evaluating the viral replication and testosterone and angiotensin levels, testis fragments were sampled and snap-frozen in liquid nitrogen. Likewise, for histological, transmission electron microscopy (TEM), and immunohistochemistry analyses, testis specimens were embedded in Methacrylate, Epon 812 resin, and Paraplast® F.
The authors tested collected samples for the presence of SARS-CoV-2 viral RNA by quantitative reverse transcriptase-polymerase chain reaction (RT-qPCR) with primers to amplify the envelope (E) gene. Samples showing a cycle threshold (CT) ≤ 40 were considered SARS-CoV-2-infected.
The control group comprised six patients who underwent orchiectomy due to prostate cancer suspicion, and their testicles were collected and used for TEM, histological, hormonal, and molecular analyses. The average age of test and control group patients was 63.9 ± 13.11 years and 58 years, respectively. None of the patients had a clinical history of previous testicular disorders.
Study findings
The RT-qPCR revealed the presence of SARS-CoV-2 RNA in the testes of 10 of the 11 patients. A nano-designed sensor using localized surface plasmon resonance (LSPR) also detected the SARS-CoV-2 spike (S) protein in the testes of 10 of the 11 patients. Prominent S-protein immunolabeling was observed in testes of all COVID-19 patients, suggesting that the SARS-CoV-2 tropism for testes was higher than previously assessed as conventional RT-qPCR protocol detected infected testes with a higher viral load only. The authors recommended developing sensitive techniques with improved detection sensitivity for the reliable detection of SARS-CoV-2 (even in a low viral titer) in testes.
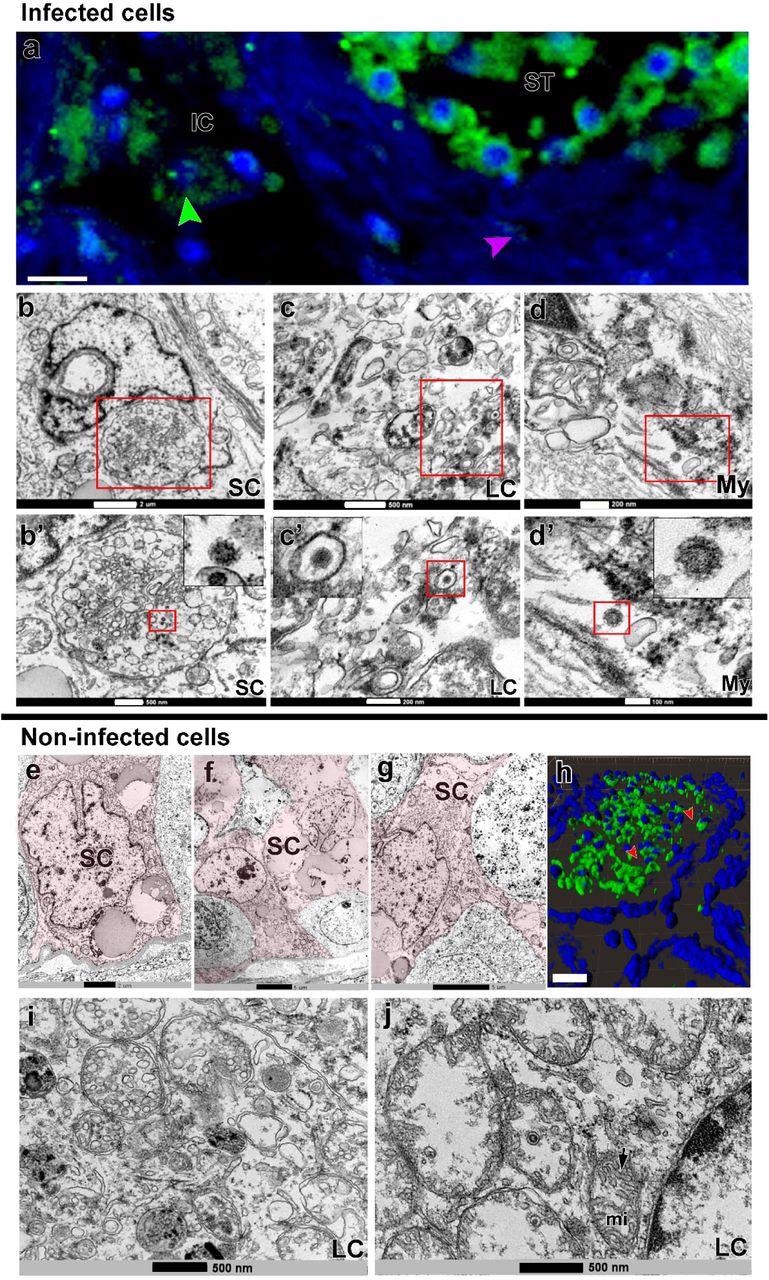
SARS-CoV-2 infection in Sertoli, Leydig and peritubular myoid cells. a) Immunofluorescence against S-protein evidencing weak labeling in peritubular myoid (pink arrowhead) and Leydig cells (green arrowhead) (Scale bar =15µm). b-d’) TEM images showing viral particles (in low and high magnification) in Sertoli cell (SC) (Scale bars = b: 2µm; b’: 500nm); Leydig cell (LC) (Scale bars = c: 500nm; c’: 200nm and peritubular myoid cell (My) (Scale bars = d: 200nm; d’: 100nm). e-g) TEM images of non-infected Sertoli cells (SC, pink) (Scale bars = e: 2µm f-g: 5µ). h) 3D reconstruction of a seminiferous tubule cross-section showing non-labeled areas surrounding germ cells (red arrowheads) (Scale bar = 40µm). i-j) high magnification of non-infected Leydig cells. Arrow = tubular crest of a mitochondria (mi) (Scale bars = 500nm). Immunofluorescence images in the testis of patient #8. TEM images in testes from patients #1, #7 and #8.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
TEM data showed several infected monocytes/macrophages surrounding blood vessels and migrating to the testis parenchyma, suggesting that these cells might be delivering SARS-CoV-2 to the testis contributing to infection of testicular cells. Most S-protein labeling was identified inside the seminiferous tubules, mainly in germ cells, increasing the concerns of potential sexual transmission, reinforced by the detection of SARS-CoV-2 RNA in the semen of critically ill COVID-19 patients.
The TEM analysis also showed that SARS-CoV-2 was replicating inside macrophages, expressing ACE2 and transmembrane protease, serine 2 (TMPRSS2), and in spermatogonial cells. SARS-CoV-2 replication complexes were visible with replication membranous webs (RMW) containing double-membrane vesicles (DMV) and Endoplasmic Reticulum Golgi Intermediate Complex (ERGIC) showing new virions. The findings suggested that migrating infected monocytes/macrophages might be transporting SARS-CoV-2 from the lungs into the testes. Due to testicular immune tolerance, viral removal/clearance from this site of the human body was difficult.
The presence of lymphocytes (CD3+) in the testes of COVID-19 patients suggested a prolonged infection. Intriguingly, SARS-CoV-2 was detected in the testis of patient 1 who died 26 days after symptom onset, thus suggesting that the testes may serve as a SARS-CoV-2 reservoir, maintaining infective virions for prolonged periods.
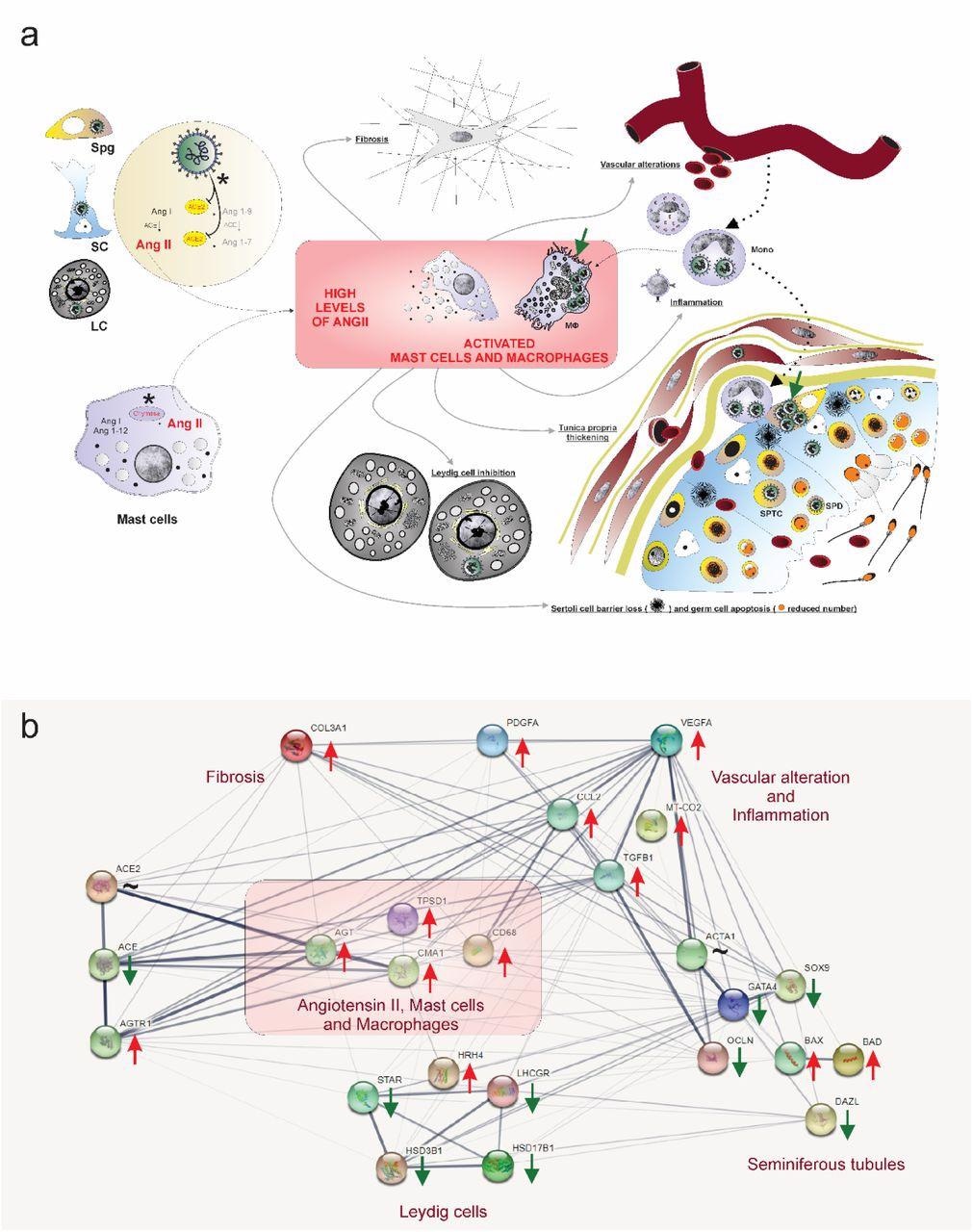
Hypothetical viral and molecular mechanisms of testis infection and damaging by SARS-CoV-2. a) SARS-CoV-2 (green color) was identified in spermatogonial cells (Spg), Sertoli cells (SC), Leydig cells (LC), infiltrative monocytes (Mono), macrophages (MΦ), spermatocytes (sptc), and spermatids (sptd). Note viral factories in macrophages and spermatogonial cells (green arrows). Direct influence of SARS-CoV-2 in testicular cells hampers ACE2 activity, while activation of mast cells (chymase positive) elevates the levels of angiotensin II (a potent pro-inflammatory molecule) (asterisks). Angiogenic and inflammatory factors can induce the infiltration and activation of mast cells. High levels of angiotensin II, activation of mast cells, and inflammatory factors can activate (polarize) macrophages. The testicular phenotype of COVID-19 patients (fibrosis, vascular alteration, inflammation, tunica propria thickening, Sertoli cell barrier loss, germ cell apoptosis, and inhibition of Leydig cells) can be linked to elevated angiotensin II and active mast cells and macrophages. b) genes network related to angiotensin II, activated mast cells, and macrophages (pink box) extracted from STRING (https://string-db.org/). These three elements up-regulate the inflammatory, apoptotic, fibrotic, and vascular genes while down-regulating critical seminiferous tubule and Leydig cell genes. Red arrows: up-regulated genes; Green arrows: down-regulated genes; ∼: genes up-and down-regulated depending on the phase.
Conclusions
To summarize, the study findings could contribute to a better understanding of SARS-CoV-2 tropism, biology, and impact on testes and male fertility.
SARS-CoV-2 infection elevated the angiotensin II levels in testicular cells of COVID-19 patients, which activated mast cells and macrophages. Subsequently, the testes of COVID-19 patients showed fibrosis, vascular alteration, inflammation, tunica propria thickening, Sertoli cell barrier loss, germ cell apoptosis, and inhibition of Leydig cells. Further, the intratesticular testosterone levels in testes of COVID-19 patients decreased 30 times. In addition, vasoconstrictive peptides fluctuated in the testes of COVID-19 critically ill patients.
Taken together, these findings suggest that testes should not be neglected while evaluating a COVID-19 patients' clinical condition because it is a site of active viral replication and a potential source of viral load.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
SARS-CoV-2 infects, replicates, elevates angiotensin II and activates immune cells in human testes, Guilherme M.J. Costa, Samyra M.S.N. Lacerda, André F.A. Figueiredo, Natália T. Wnuk, Marcos R. G. Brener, Gabriel H. Campolina-Silva, Andrea Kauffmann-Zeh, Lucila GG Pacifico, Alice F. Versiani, Lídia M. Andrade, Maísa M. Antunes, Fernanda R. Souza, Geovanni D. Cassali, André L. Caldeira-Brant, Hélio Chiarini-Garcia, Vivian V. Costa, Flavio G. da Fonseca, Maurício L. Nogueira, Guilherme R. F. Campos, Lucas M. Kangussu, Estefânia M. N. Martins, Loudiana M. Antonio, Cintia Bittar, Paula Rahal, Renato S. Aguiar, Bárbara P. Mendes, Marcela S. Procópio, Thiago P. Furtado, Yuri L Guimaraes, Gustavo B Menezes, Ana Martinez-Marchal, Miguel Brieno-Enriquez, Kyle E. Orwig, Marcelo H. Furtado, medRxiv 2022.02.05.22270327; doi: https://doi.org/10.1101/2022.02.05.22270327, https://www.medrxiv.org/content/10.1101/2022.02.05.22270327v1
- Peer reviewed and published scientific report.
Costa, Guilherme M. J., Samyra M. S. N. Lacerda, André F. A. Figueiredo, Natália T. Wnuk, Marcos R. G. Brener, Lídia M. Andrade, Gabriel H. Campolina-Silva, et al. 2023. “High SARS-CoV-2 Tropism and Activation of Immune Cells in the Testes of Non-Vaccinated Deceased COVID-19 Patients.” BMC Biology 21 (1). https://doi.org/10.1186/s12915-022-01497-8. https://bmcbiol.biomedcentral.com/articles/10.1186/s12915-022-01497-8.