Neutralizing antibodies have been widely used to treat coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). However, this treatment approach is associated with several limitations, including the inability to sterilize the mucosa from where viral shedding occurs, the emergence of antibody-resistant variants, and high costs.
Study: Human inhalable antibody fragments neutralizing SARS-CoV-2 variants for COVID-19 therapy. Image Credit: Huen Structure Bio / Shutterstock.com
Introduction
Initially, single domain nanobodies based on the variable region of the heavy chain of anti-SARS-CoV-2 antibodies were considered a possible therapeutic avenue for use by inhalation as compared to systemic monoclonal antibodies. These nanobodies are of camelid origin and must therefore be humanized through processes that may be expensive and reduce their scope.
The current study focuses on small human antibodies generated by engineering immunoglobulin (Ig) variable light and heavy chain sequences together, forming a single small polypeptide through the use of a flexible peptide linker. The linker allows the functional antigen-binding domains to be re-formed into single-chain fragment variable (scFv) antibodies.
Notably, scFv antibodies do not contain the crystallizable fragment (Fc) of the antibody molecule and, as a result, do not cause antibody-dependent enhancement of disease (ADE). Furthermore, scFv antibodies also do not require expensive mammalian expression systems and can be rapidly produced on a large scale, thus making them much cheaper to produce than conventional monoclonal antibodies.
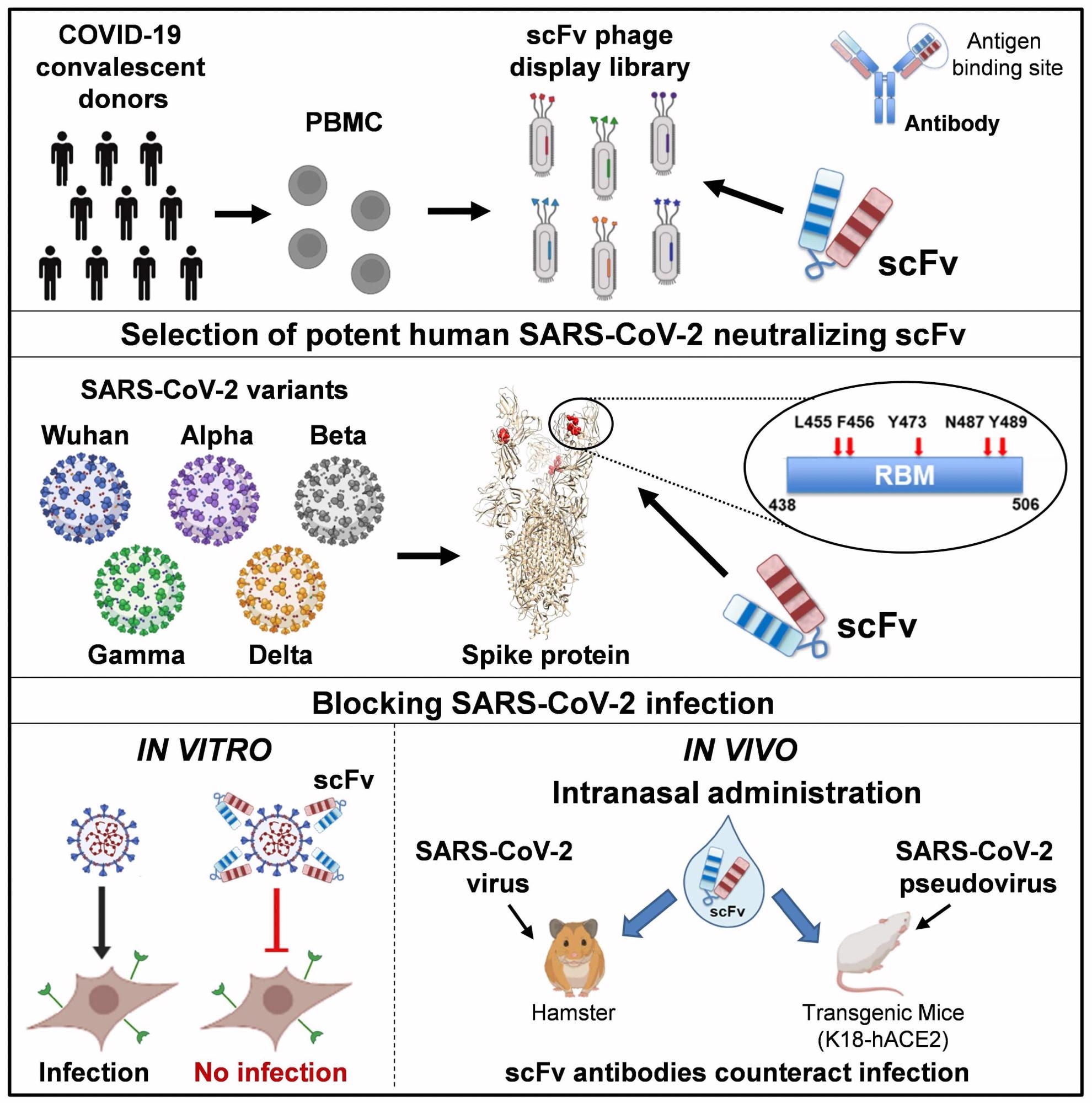
These non-glycosylated simple polypeptides are small and simple molecules that are highly stable. Of the range of antibodies, those that showed reactivity with the SARS-CoV-2 spike S1 and receptor-binding domain (RBD) proteins of the SARS-CoV-2 Delta and other variants being monitored (VBMs) were selected for their binding, affinity, and inhibition. Most of the mutations present in the currently known variants of concern were included.
All spike and RBD variants were efficiently recognized and bound by scFv76-cluster antibodies (76clAbs), comprising the affinity matured derivatives of scFv76. The affinity of scFv76 was superior to that offered by scFv5 and scFv86 antibodies, which is likely due to the different epitopes of the latter two antibodies. Surface Plasmon Resonance (SPR) results also indicated that 76clAbs binds to the same or very close epitopes, whereas scFv5 binds to another epitope.
All 76clAbs recognize the native SARS-CoV-2 spike protein and block its binding with the human angiotensin-converting enzyme 2 (hACE2) receptor, but not the other scFv antibodies. Except for scFv76-55, all 76clAbs accommodated Delta variant mutations and other VBM mutations without losing their neutralizing activity.
The 50% inhibitory concentrations (IC50) fell between 0.36 to 4.30 nanomolar (nM), which was in contrast to the greater than 40 nM concentrations, below which scFv5 and scFv86 allowed spike or RBD binding to the receptor.
With cell infection experiments, 76clAbs suppressed the infection of pseudoviruses bearing the Delta spike, D614G spike, or the spike proteins of several variants of concern (VOCs) and VBMs. Again, the lowest neutralizing activity was with scFv76-55, which showed the greatest loss of activity with mutations in the RBD.
Switching to experiments using the authentic viral variants, the scientists found that all 76clAbs also suppressed the cytopathic effect (CPE) with a 50% microneutralization titer (MN50) of less than 15 nM against the Delta variant.
When these were added one hour after inoculation of the cell culture with any of the SARS-CoV-2 variants, including the ancestral Wuhan, D614G, and Alpha strains, effective inhibition of all variants occurred. The IC50 was less than 22 nM.
With pretreatment one hour before virus inoculation, the IC50 was less than a tenth of this. The same results were seen using pooled immune sera from six COVID-19 convalescent donors, showing a wide gap between the IC50 for pretreatment and post-treatment of inoculated samples.
These antibodies also disrupted spike-induced membrane fusion that mediates viral entry into the host cells. This is considered an important property, as pulmonary syncytia formation appears to be the characteristic lesion of this infection and is largely responsible for localized inflammation and thromboembolism. However, at nanomolar concentrations, scFv76 blocked fusion with the three variants mentioned above.
The 76clAbs also bound to an epitope on the receptor-binding motif (RBM); however, the scFv86, which is a non-neutralizing antibody, recognizes another epitope with the critical residue lying outside the RBM. The 76clAb-binding epitope includes residues F456, Y473, N487, and Y489, none of which are mutated in the Delta variant, any VBM spike variant, or even the Omicron variant, despite the latter SARS-CoV-2 strain having the highest number of RBD mutations.
In fact, Omicron evades neutralizing antibodies in immune sera and most anti-RBD monoclonal antibodies. Yet, all residues remained intact, thereby indicating the highly conserved nature of this epitope.
This cluster of antibodies contains two species in monomeric and dimeric scFv76 forms, with the most stable being scFv76, scFv76-46, and scFv76-58. At 90 °C, the folded beta-sheet structure of these proteins unfolds. Thus, these antibodies are amenable to nebulization without losing their integrity, proteinaceous nature, or ability to bind the SARS-CoV-2 spike protein.
Intranasal administration of scFv76, at 37 μg/nostril in a transgenic mouse model completely suppressed viral infection when given two hours before or four hours after infection. Hamsters also were protected against clinical disease when comparable doses were given two hours before and then once daily for two days from the infection.
Further testing showed that in the nebulized form, scFv76 can be effectively delivered into the alveolar spaces, with the amount deposited building up over time.
Implications
Here we describe a class of anti-SARS-CoV-2 human scFv antibodies with subnanomolar neutralization potency, in vivo efficacy and significant stability. These small size fully human antibodies are promising candidates for early treatment of COVID-19 as a complementary approach to the existing vaccination and passive systemic immunization, which do not induce a robust mucosal defense.”