The emerging SARS-CoV-2 variants of concern (VOCs) and their related transmissibility have worsened the impact of the coronavirus disease 2019 (COVID-19) pandemic. The development of therapeutic drugs against the virus requires the knowledge of indispensable mutations in the new VOCs that can be targeted for inhibition.
 Study: Crippled Coronavirus: 5′-PolyU targeted Oligo prevents development of infectious Virions. Image Credit: unoL / Shutterstock
Study: Crippled Coronavirus: 5′-PolyU targeted Oligo prevents development of infectious Virions. Image Credit: unoL / Shutterstock

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
The present study hypothesized the inhibition of the 5’- polyuridine (polyU) tract of the viral antigenome using complementary oligonucleotides to limit the generation of ribonucleic acid (RNA) viruses.
The team developed two oligonucleotides using genomic sequences of mouse hepatitis virus (MHV)-A59 strain, which is the prototype strain for CoV. These oligonucleotides were complementary to the polyUs present at the 5’ end of the minus strand of the viral genome. The designed oligonucleotides had 21 3’-polyadenylic acids (polyA) and 11 bases, which were complementary to the flanking polyAs, which helped the oligonucleotides distinguish between the 5’-polyUs and the 3’-polyUs in the genome. A frameshift mutation was also produced to determine the abilities of oligonucleotides as a primer.
The oligonucleotides were complexed and transfected to the infected cells using cationic polymers. Complexation of oligonucleotide was performed via electrostatic interactions of the
deoxyribonucleic acid (DNA) backbone with the delivery system. The delivery vehicle for the oligonucleotide was formulated with either a polymer-based or lipid-based delivery agent. The study also involved control cells which were treated only with a polymer. The development of infectious virions based on primary infection of oligonucleotides was assessed by examining any infectious effects induced by the virus in the secondary cells.
The team then investigated the potential of oligonucleotides in preventing virion generation in the cells by isolating virion proteins from the treated and the control cells. The western blot was then used to detect the presence of viral nucleocapsid (N) protein. Finally, the infection potential of the virions released by the oligonucleotide-treated cells was analyzed using the released virions detected in the media.
Furthermore, replicating virions present in the oligonucleotide-treated cells were detected using assays for replicase-transcriptase complexes (RTCs), which are characteristically found in virus-infected cells. This assay was based on the detection of double-stranded-RNA (ds-RNA) intermediaries which were considered as a marker for the synthesis of viral RNA in infected cells.
Results
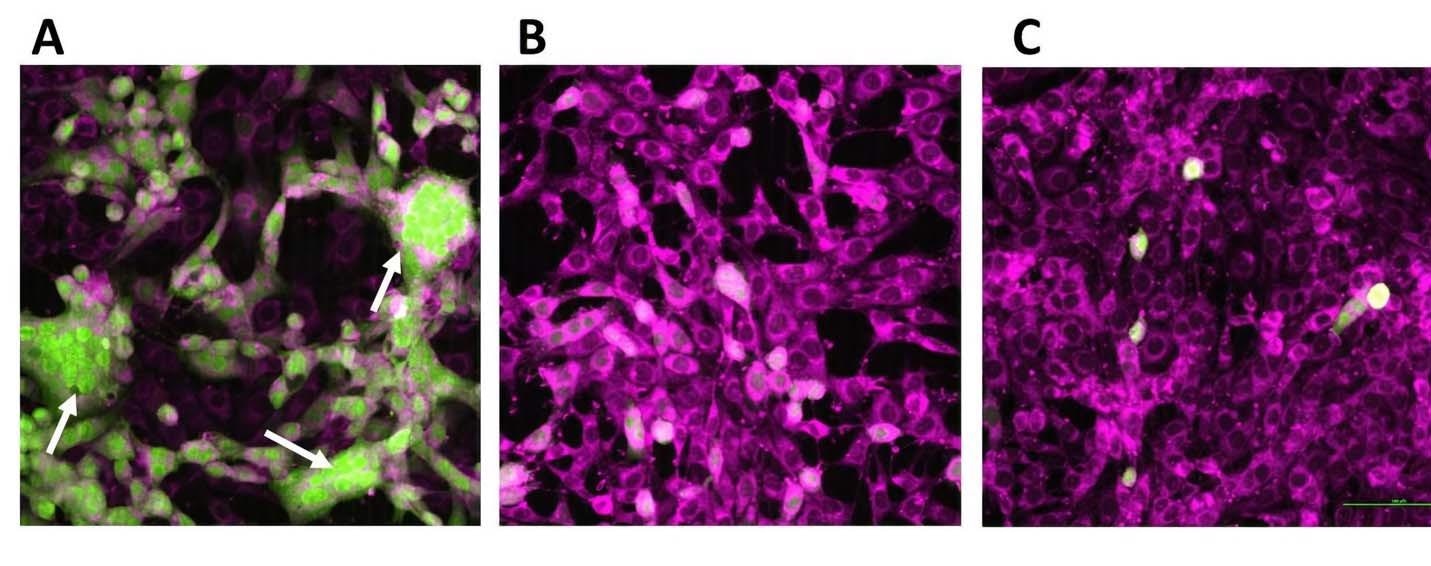
The study results showed that the control cells exhibited clear infectiousness due to the virus, with infections concentrated around specific foci. Also, the formation of a syncytium due to many cell-to-cell fusions was observed, which indicated extensive virion production in the secondary cells.
Cells treated with oligonucleotides had a few green fluorescent proteins (GFP)-positive secondary cells, while rare formations of syncytium were also found. However, some secondary cells did show GFP-positivity in the presence of oligonucleotide without any cytopathic effect or any syncytium formation. This suggested that cells treated with oligonucleotides did not produce any fully formed functional virions.
The western blot showed that the released virions showed an extensive generation of N-proteins in the control cells. At the same time, insignificant levels of N-proteins were found in oligonucleotide-treated cells. Moreover, media of the polymer-treated cells did not have any N-protein, while media obtained from oligonucleotide-treated cells showed only a slightly lesser amount of N-proteins. Overall, it was observed that cells treated with oligonucleotide were able to inhibit the formation of proteins essential for the production of functional virions. Background resolution of the western blots found higher molecular weight bands produced in the control cells while no such bands were present in the oligonucleotide-treated cells.
On analyzing the virions obtained from the media of the oligonucleotide-treated and the control cells, it was found that the media of the polymer-treated cells contained functional virions that caused a cytopathic effect in the secondary cells after only six hours of addition to the secondary cells. On the other hand, media from the oligonucleotide-treated cells had no cytopathic effects in the early phase of infection. Furthermore, post 24 hours, the media of the control cells were GFP-positive in almost all the secondary cells, while only a few secondary cells were positive for the presence of infectious virions in the media of oligonucleotide-treated cells.
Investigation of RNA synthesis processes in the oligonucleotide-treated cells showed that the oligonucleotide efficiently inhibited the generation of ds-RNA intermediaries while the control cells indicated active infection with the development of the intermediaries.
Conclusion
The study findings showed that oligonucleotides could successfully target the 5’-polyUs in the mouse coronavirus genome. The researchers believed that developing therapeutic drugs against COVID-19 using indispensable mutational targets like 5’-polyU tract can help mitigate the generation and transmission of emerging SARS-CoV-2 VOCs.

 *Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: bioRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.