Since the onset of the coronavirus disease 2019 (COVID-19) pandemic, the causative severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has undergone several genomic mutations.
The World Health Organization (WHO) has classified the newly emergent variants of SARS-CoV-2 as variants of concern (VOC) and variants of interest (VOI) according to their virulence and transmissibility. Currently, the SARS-CoV-2 Omicron VOC is the dominant circulating strain in many countries around the world.

Study: Increased receptor affinity and reduced recognition by specific antibodies contribute to immune escape of SARS-CoV-2 variant Omicron. Image Credit: Vinayak Jagtap / Shutterstock.com

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
The SARS-CoV-2 Omicron variant (B.1.1.529) was first reported in South Africa in November 2021. Soon after, the Omicron variant was detected in 135 other countries around the world. This caused the WHO to declare that the emergence of the Omicron variant increased the global risk of perpetuating the COVID-19 pandemic.
Preliminary studies that compared the Omicron variant with other VOCs have reported that an increased risk of reinfection is associated with infection with the Omicron variant. One of the primary concerns associated with the Omicron variant is its ability to infect individuals who have received two doses of current COVID-19 vaccines. However, the Omicron predominantly causes relatively mild infection. Therefore, despite an overwhelming surge in the number of COVID-19 cases, healthcare systems have remained functional.
Previous studies have reported that compared to the original SARS-CoV-2 strain, the Delta variant harbors seven mutations in the spike protein, including two mutations (L452R and T478K) in the receptor-binding domain (RBD). However, this variant does not contain the E484 mutation, which is strongly related to the reduced recognition by antibodies.
A considerable number of mutations have been identified in the Omicron variant as compared to other VOCs, with most mutations residing in the spike domain. Importantly, this variant contains a mutation in the RBD region that interacts with the angiotensin-converting enzyme 2 (ACE2) cell receptor. Additionally, previous studies have also reported the presence of the E484A mutation, which is responsible for the altered antibody recognition of Omicron.
All current COVID-19 vaccines and therapeutics have been designed against the spike protein. Thus, the effectiveness of the existing vaccines against the Omicron variant, which has been determined through computational in silico modeling, has revealed the ability of the Omicron variant to evade antibody-mediated immunity.
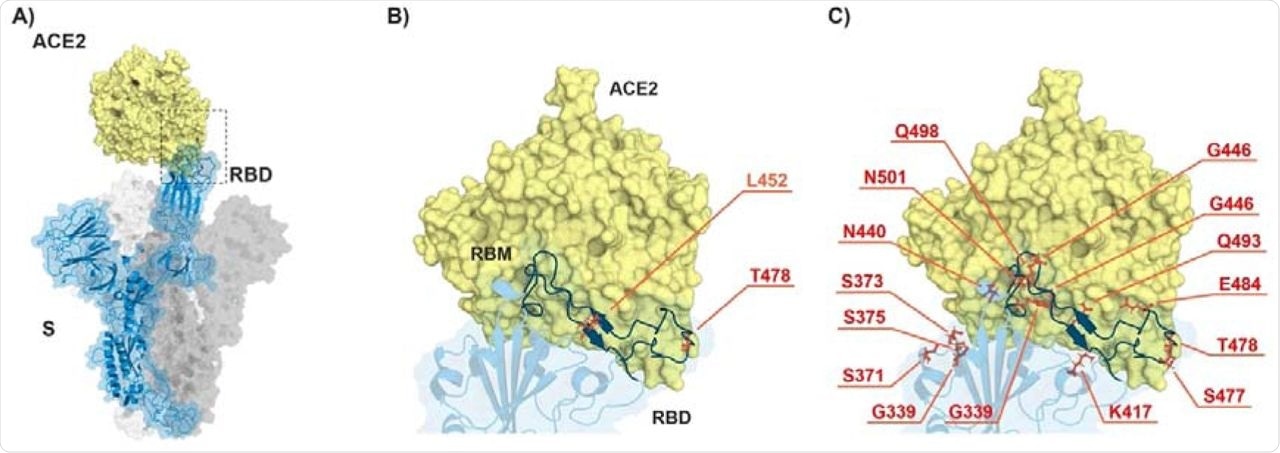
ACE2-spike interaction and mutations found in RBD of B.1.617.2 (Delta) and B.1.1.529 (Omicron). A) S monomer (blue ribbon and surface) bound to ACE2 ectodomain (yellow surface). B) Detail of A), highlighting the mutated residues (orange sticks) in Delta. C) Same as in B) but highlighting the mutated residues in Omicron. From PDB files 6ACG and 2AJF.
About the study
Some of the key parameters that have contributed to the global impact of the SARS-CoV-2 Omicron variant include its infectiousness and virulence, as well as the effectiveness of current vaccines against this variant and the degree of awareness of the general population in adopting protective measures. A new study published on the bioRxiv* preprint server reveals the underlying mechanism associated with the Omicron variant’s increased transmissibility and ability to evade immune responses induced by either vaccination or natural infection.
In this study, the authors obtained human sera within one-month post-vaccination from COVID-19 convalescent patients, vaccinated individuals with two doses of a messenger ribonucleic acid (mRNA) vaccine, and vaccinated individuals who had received three doses of an mRNA vaccine. The study candidates were employed at the University Hospital of Bern in Switzerland.
Study findings
Researchers conducted Biolayer Interferometry (BLI) and revealed that at the biophysical level, both RBD-Omicron and RBD-Delta have increased affinity to ACE2 as compared to the original SARS-CoV-2 RBD. Consistent with previous studies, the researchers reported that the E484A mutation accounts for the ability of the Omicron variant to evade immune responses by altering an important epitope for neutralizing antibodies.
The enhanced affinity of the Omicron RBD for ACE2 represents a challenge for neutralizing antibodies. Moreover, the Omicron variant binds with the ACE2 in a way that prevents antibodies from competing with and blocking this variant. This phenomenon is known as ‘affinity escape,’ which is different from the classical antibody specificity escape mechanism.
Current COVID-19 vaccines could provide better protection if they were optimized to produce a higher number of antibodies with a greater affinity for ACE2. Importantly, the booster vaccination strategy has greatly contributed to this type of approach; therefore, individuals who have received three doses of mRNA vaccines are relatively better protected from the Omicron strain as compared to those who have only received two vaccine doses.
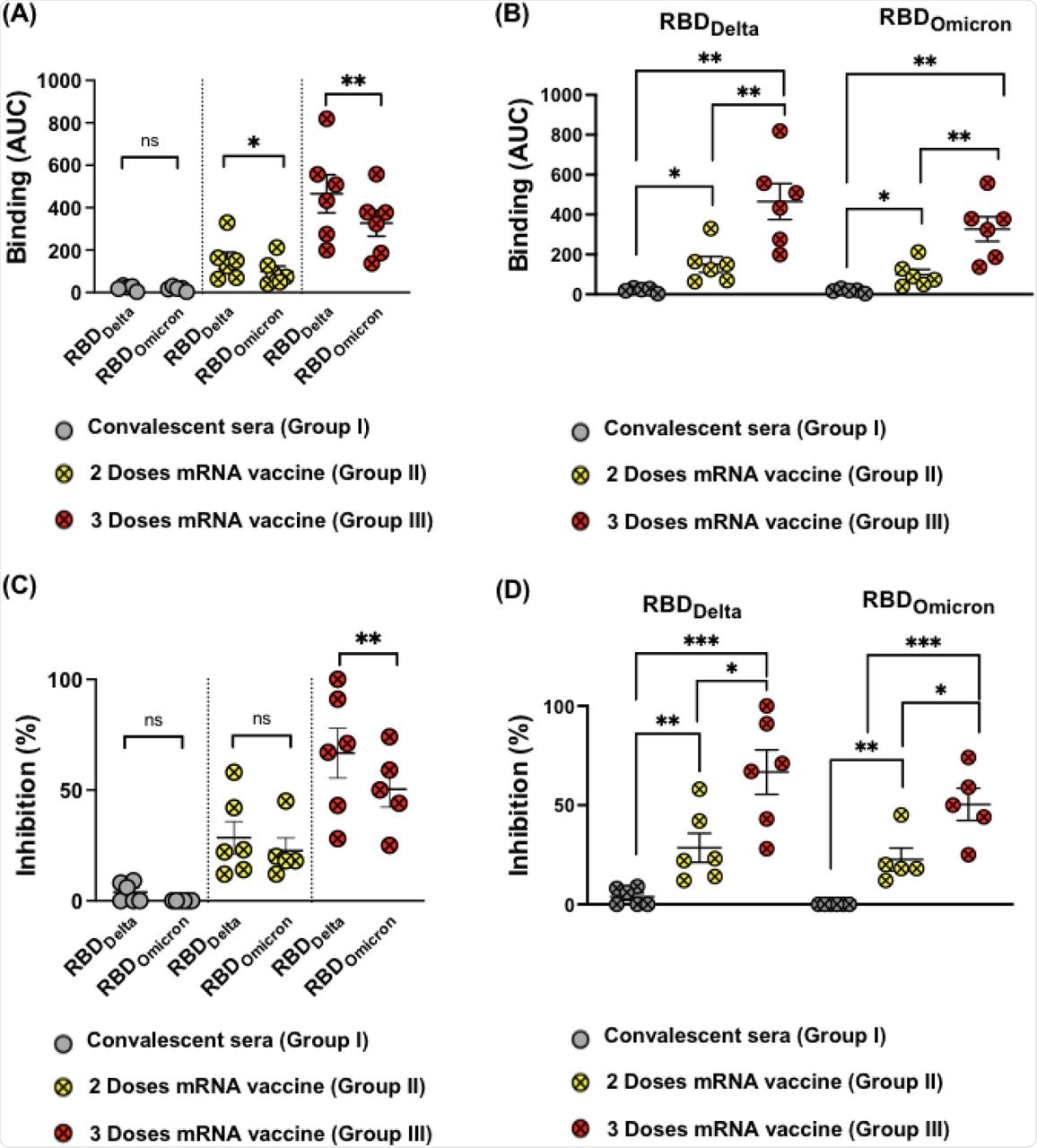
(A-B) Binding of sera from (Group I, II and III) to RBDWT, RBDDelta and RBDOmicron using BLI, depicted as AUC. (C and D) Inhibition of RBDWT, RBDDelta and RBDOmicron binding to the human receptor ACE2 by 1:80 sera dilution, sera from the three different groups were used in this assay. Statistical analysis by Student’s t-test, (Paired in A and C) and (Unpaired in B and D). N= 6 or 5. One representative of two similar experiments is shown.
Conclusions
The authors observed enhanced affinity of the Omicron variant for the ACE2 receptor and reduced recognition by serum antibodies. Moreover, the underlying mechanisms associated with the immune escape capabilities of the Omicron variant include its altered antibody specificity and high RBD-ACE2 affinity that outcompetes antibody binding.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Vogt, C. A., Augusto, G., Martina, B., et al. (2022) Increased receptor affinity and reduced recognition by specific antibodies contribute to immune escape of SARS-CoV-2 variant Omicron. bioRxiv. doi:10.1101/2022.03.11.483934. https://www.biorxiv.org/content/10.1101/2022.03.11.483934v1.
- Peer reviewed and published scientific report.
Vogt, Anne-Cathrine S., Gilles Augusto, Byron Martina, Xinyue Chang, Gheyath Nasrallah, Daniel E. Speiser, Monique Vogel, Martin F. Bachmann, and Mona O. Mohsen. 2022. “Increased Receptor Affinity and Reduced Recognition by Specific Antibodies Contribute to Immune Escape of SARS-CoV-2 Variant Omicron.” Vaccines 10 (5): 743. https://doi.org/10.3390/vaccines10050743. https://www.mdpi.com/2076-393X/10/5/743.