The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by the rapid outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has claimed more than 6.1 million lives worldwide. Scientists have worked at a record speed to understand various aspects of SARS-CoV-2, as well as develop effective vaccines and therapeutics to protect individuals from COVID-19.
Background
The SARS-CoV-2 spike protein binds with the angiotensin-converting enzyme 2 (ACE2) receptor, which is expressed by various cell types, including the nasal epithelia, to establish infection. The conjunctival goblet cell serves as an alternative infection pathway.
It is imperative to study the mucosal antibodies of COVID-19 patients to understand viral-host interaction and the underlying immunopathology. To date, very little evidence is available on SARS-CoV-2-specific antibodies of the conjunctival and respiratory mucosae.
Previous studies have reported that mucosal immunity is gained by innate and acquired immune responses. When the SARS-CoV-2 antigen is acquired locally in the nasal epithelia, it is processed in the nasopharyngeal-associated lymphoid tissue (NALT).
Likewise, when the viral antigen is locally acquired in the conjunctival tissue, it is processed by the conjunctiva-associated lymphoid tissue (CALT). These lymphoid tissues generate immunoglobulin A (IgA)-producing mucosal B-cells, which express receptor proteins for effective trafficking to the mucosal effector site.
Researchers have revealed that secretory IgA, which is a dimeric form of IgA, is present on mucosal surfaces and provides wide-ranging protection owing as a result of its high avidity. Moreover, secretory IgA influences the agglutination and neutralization of viruses within the lamina propria beneath the epithelium, respiratory tract, and respiratory cell. Importantly, this dimeric IgA provides better protection against COVID-19 as compared to the IgG isotype.
Previous studies have determined high levels of serological IgA in SARS-CoV-2 infected patients. In fact, even mild to moderate SARS-CoV-2 infection with low antigenic exposure appears to be capable of stimulating a mucosal SARS-CoV-2-specific IgA response.
This could be accompanied by the absence, presence, or delay of systemic virus-specific IgA production. Taken together, this pattern has been observed in young individuals and children with asymptomatic or mild SARS-CoV-2 infection.
About the study
The current longitudinal study aimed to determine mucosal SARS-CoV-2-specific antibody levels and their neutralizing effect in both children and adults infected with SARS-CoV-2. In this study, conjunctival fluid (CF) samples were collected using methods similar to Schirmer’s test, whereas nasal epithelial lining fluid (NELF) samples were obtained using nasal strips.
All samples remained stable at room temperature, which is advantageous over current mucosal sampling methods for nasal swabs or irrigation. Both pediatric and adult COVID-19 patients were included in the current study. To this end, their S1-specific mucosal antibody levels were assessed longitudinally from hospital admission to six months post-diagnosis of SARS-CoV-2 infection.
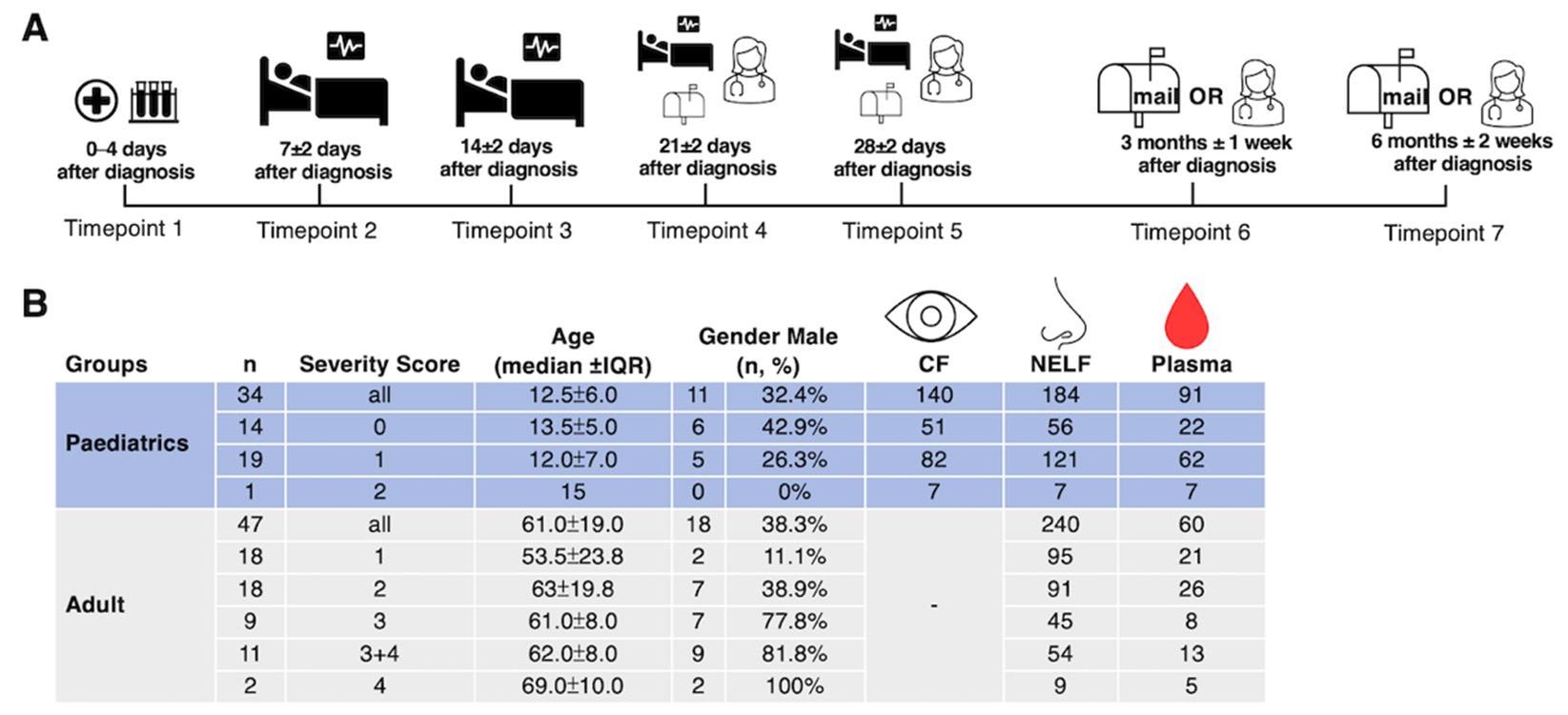 Study design and demographics. (A) A longitudinal sample collection, from the day of diagnosis (disease onset or the first day of a SARS-CoV-2 PCR positive result, whichever was earlier) to six months post-diagnosis, was conducted by healthcare workers during hospitalization and follow-up consultations for pediatric patients. Adult patients performed the self-collection of NELF samples after being discharged and mailed the samples to the laboratory. (B) The number of asymptomatic and symptomatic pediatric and adult subjects, their severity score (0: asymptomatic; 1: mild; 2: moderate; 3: severe; 4: critically ill), age, gender, and the number of CF, NELF and plasma samples collected are shown.
Study design and demographics. (A) A longitudinal sample collection, from the day of diagnosis (disease onset or the first day of a SARS-CoV-2 PCR positive result, whichever was earlier) to six months post-diagnosis, was conducted by healthcare workers during hospitalization and follow-up consultations for pediatric patients. Adult patients performed the self-collection of NELF samples after being discharged and mailed the samples to the laboratory. (B) The number of asymptomatic and symptomatic pediatric and adult subjects, their severity score (0: asymptomatic; 1: mild; 2: moderate; 3: severe; 4: critically ill), age, gender, and the number of CF, NELF and plasma samples collected are shown.
Study findings
The longitudinal profiling of the antibodies revealed a dominance of IgA mucosal response in COVID-19 patients. In the study cohort, none of the pediatric patients showed clinical symptoms or any indication of conjunctivitis.
An increased level of IgA was observed from zero to four days and 12 to 16 days post COVID-19 diagnosis in the symptomatic patients. As compared to asymptomatic patients, symptomatic patients exhibited significantly higher IgA levels in the second and fourth weeks post-diagnosis. However, the authors did not detect any IgG in CF samples.
These findings imply a robust involvement of CALT in symptomatic pediatric patients, which might be due to an anterior chamber-associated immune deviation that tends to abolish B-cells.
Importantly, 70% of the CF from symptomatic patients was found to remain IgA-positive, even after six-months post-COVID-19 diagnosis as compared with 43% in asymptomatic children. An opposite pattern was observed in the nasal mucosa samples.
The researchers further reported that the mucosal IgA response was localized. Thus, if IgA were systematically produced, it was not transported into the secretions.
In comparison to pediatric patients with mild disease, asymptomatic pediatric patients revealed induction of an early and robust nasal epithelial lining fluid (NELF) IgA.
All adults exhibited reduced NELF IgA levels from zero to four days post-diagnosis; however, the first statistically significant IgA level was detected 12 to 16 days post-diagnosis. Additionally, a smaller number of adult patients with mild disease, as compared to severely or critically infected patients, showed higher levels of IgA in the plasma at five to nine days post-diagnosis.
The current mucosal antibody test could thus be effectively used for the early detection of COVID-19 in asymptomatic individuals. Furthermore, NELF IgA remained detectable for at least 50% of the COVID-19 patients three months post-diagnosis.
Limitations
One of the key limitations of this study is the determination of SARS-CoV-2-specific antibodies based on only IgA and IgG isotypes and those against S1. Thus, the diversity of the antibody responses to other SARS-CoV-2 viral antigens was not investigated.
It is extremely important to evaluate antibody diversities in mucosal fluids and analyze their immunological relevance in varied disease outcomes. Another limitation was the absence of a cell-based plaque reduction assay.
Journal reference:
- Chan, R. W. Y., Chan, K. C. C., Lui, G. C. Y., et al. (2022) Mucosal Antibody Response to SARS-CoV-2 in Paediatric and Adult Patients: A Longitudinal Study. Pathogens 11(4). doi:10.3390/pathogens11040397