As of April 17, 2022, over 504 million coronavirus disease 2019 (COVID-19) cases have been recorded to date, with over 6.2 million fatalities. Although vaccines against SARS-CoV-2 have significantly decreased morbidity and mortality, potent therapeutic interventions are still needed to treat unvaccinated subjects and those experiencing breakthrough infections.
Direct-acting antivirals like molnupiravir and nirmatrelvir are currently approved for COVID-19 treatment. Other therapies like type I interferon (IFN) or neutralizing antibodies (nAbs) are supplementary treatments that are used to enhance host defenses.
Metabolic syndrome and hyperlipidemia have been associated with poor COVID-19 outcomes, which has led researchers to hypothesize that statins, which lower cholesterol levels, might improve survival in these individuals. Cholesterol forms a vital component of cell membrane lipids and its homeostasis is essential for viruses to enter, replicate, assemble, and egress from host cells.
Previously, the authors of the present study had reported the induction of metabolic reprogramming by ACAT inhibition to boost the exhausted T-cell responses.
Study findings
In the present study, researchers tested whether the modulation of cholesterol metabolism by Avasimibe (AVS), an ACAT inhibitor, could regulate SARS-CoV-2 replication and T-cell responses specific to the virus.
VeroE6 cells lacking transmembrane protease serine 2 (TMPRSS2) or overexpressing it (VeroE6-TMPRSS2 cells) were treated with lentiviral pseudo particles coated with the SARS-CoV-2 spike (S) protein to study cell entry. Pseudo-particle infection was reduced when cells were pre-treated with AVS. The plasma membrane of VeroE6 cells treated with AVS showed a modest increase in free cholesterol levels, whereas total levels were unchanged.
In Calu-3 cells, pseudo particle infection was inhibited in a dose-dependent manner with a half-maximal inhibitory concentration (IC50) of 1.77 micromolar (μM). AVS inhibited infection of Calu-3 and VeroE6-TMPRSS2 cells by pseudo particles displaying the S protein of SARS-CoV-2 B.1, Delta or Omicron variants. When pseudo particles expressing vesicular stomatitis virus (VSV) G glycoprotein infected Calu-3 or VeroE6-TMPRSS2 cells, AVS reduced VSV infectivity in both cell lines, thereby indicating that AVS activity perturbs endocytic pathways.
Calu-3 cells pretreated with AVS were infected with (authentic) SARS-CoV-2, which resulted in a significant decrease of intracellular viral ribonucleic acid (RNA). Further, Calu-3 or Vero-E6-TMPRSS2 cells were treated with AVS post-infection with the authentic virus, thus demonstrating a decline in SARS-CoV-2 ribonucleic acid (RNA). Inhibition of viral infection in both cell lines by AVS was comparable to that of the antiviral remdesivir.
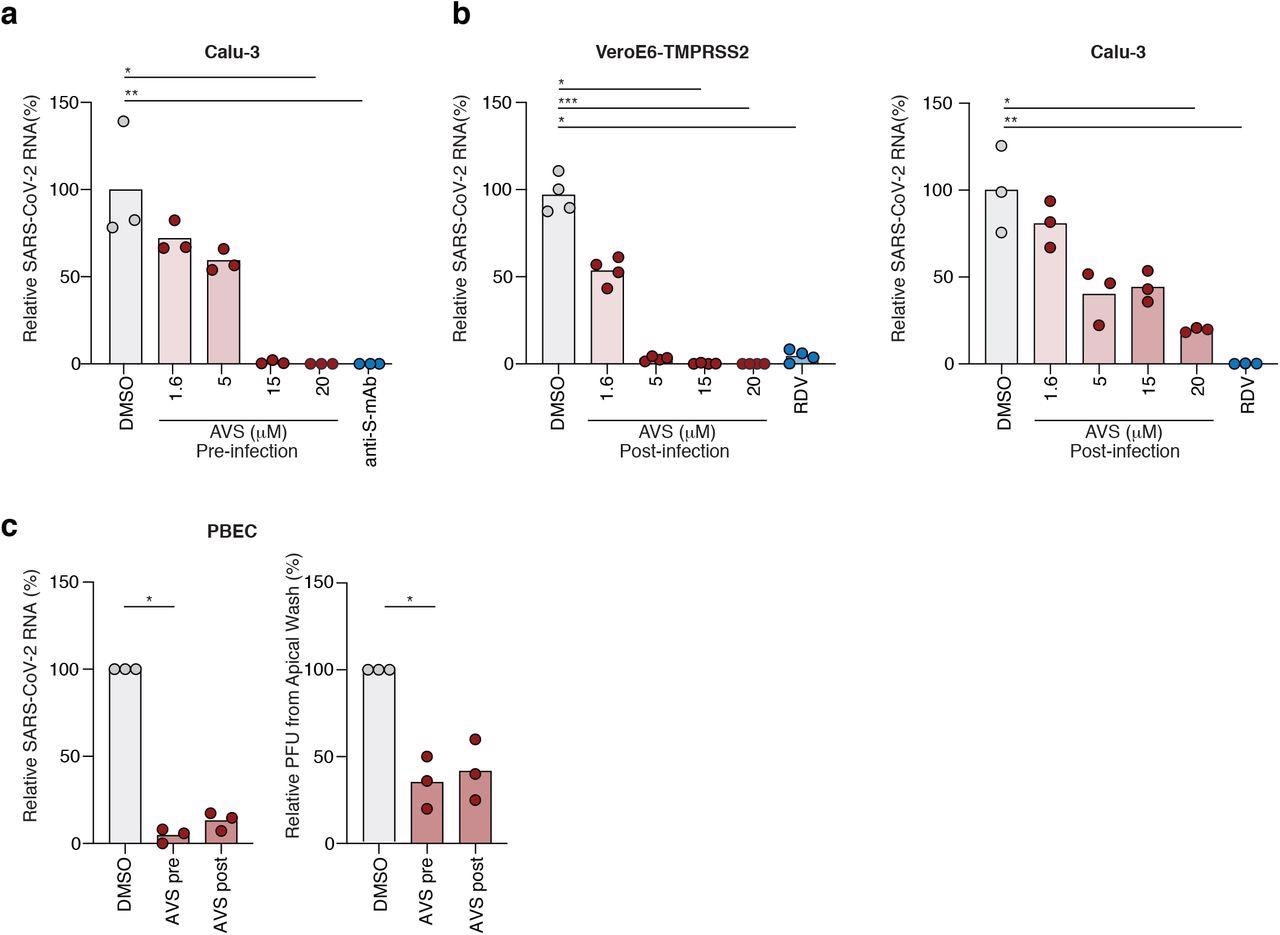 Avasimibe blocks SARS-CoV-2 entry and replication. (a) Calu-3 cells were pre-treated with AVS for 24h prior to infection with SARS-CoV-2 (VIC 01/20) at an MOI of 0.01. Cells were harvested 24h post infection and intracellular viral RNA quantified by qPCR. Data is representative of n=3-4 biological replicates. (b) VeroE6-TMPRSS2 (left) and Calu-3 (right) cells were infected with SARS-CoV-2 (MOI 0.01) for 2h, the inoculum was removed, and the cells treated with AVS. Cells were harvested 24h post infection and intracellular viral RNA quantified by qPCR. Data is representative of n=4 biological replicates. (c) Primary bronchial epithelial cells (PBEC) grown to air-liquid-interface were treated with 20μM of AVS either 24h pre- or 2h post infection of the apical surface with SARS-CoV-2 (MOI 0.1). Cultures were harvested 24h post infection and viral RNA quantified by qPCR and infectious virus shed from the apical surface by viral plaque assay. Data is representative of n=3 donors. All data are normalized to mean of DMSO and P values determined by ANOVA (Kruskal Wallis).
Avasimibe blocks SARS-CoV-2 entry and replication. (a) Calu-3 cells were pre-treated with AVS for 24h prior to infection with SARS-CoV-2 (VIC 01/20) at an MOI of 0.01. Cells were harvested 24h post infection and intracellular viral RNA quantified by qPCR. Data is representative of n=3-4 biological replicates. (b) VeroE6-TMPRSS2 (left) and Calu-3 (right) cells were infected with SARS-CoV-2 (MOI 0.01) for 2h, the inoculum was removed, and the cells treated with AVS. Cells were harvested 24h post infection and intracellular viral RNA quantified by qPCR. Data is representative of n=4 biological replicates. (c) Primary bronchial epithelial cells (PBEC) grown to air-liquid-interface were treated with 20μM of AVS either 24h pre- or 2h post infection of the apical surface with SARS-CoV-2 (MOI 0.1). Cultures were harvested 24h post infection and viral RNA quantified by qPCR and infectious virus shed from the apical surface by viral plaque assay. Data is representative of n=3 donors. All data are normalized to mean of DMSO and P values determined by ANOVA (Kruskal Wallis).
Similarly, human primary bronchial epithelial cells (PBEC) grown at the air-liquid interface were infected with SARS-CoV-2. The authors observed that pre-or post-infection AVS treatment decreased viral RNA and shedding of virus particles.
Subsequently, the effect of AVS treatment on the activity of T-cells was examined. Peripheral blood mononuclear cells (PBMCs) were obtained from unvaccinated COVID-19 patients hospitalized during the first wave in the United Kingdom between March 2020 and July 2020. PBMCs were stimulated with viral peptide pools derived from the S or membrane (M) protein with or without AVS treatment.
The team observed an increased frequency of cluster of differentiation 4 (CD4+) T-cells secreting interferon-gamma (IFN-γ), tumor necrosis factor (TNF), or both, and macrophage inflammatory protein (MIP)-1β. Some samples exhibited boosted responses, while others induced de novo responses.
While CD8+ T-cells were also similarly enhanced in response to AVS in individual donors, the overall change across the cohort was insignificant. Treatment with AVS also increased the proliferation of SARS-CoV-2-specific CD4+ T-cells.
Another cohort of unvaccinated donors was recruited six months after recovering from COVID-19 to assess whether memory T-cell responses were affected by AVS. The researchers found no significant effect on memory CD44+ and CD8+ T-cell responses.
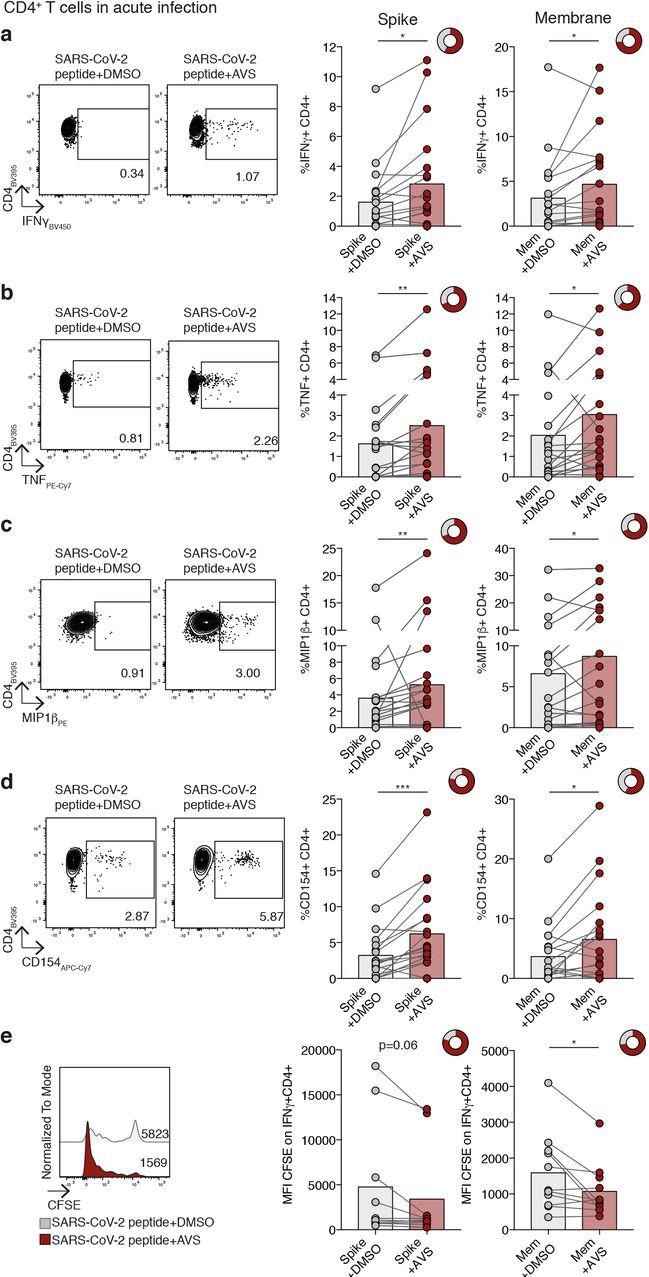
Impact of Avasimibe on SARS-CoV-2-specific CD4+ T cells in acute infection. (a-e) Human PBMC from donors with acute SARS-CoV-2 infection were stimulated with SARS-CoV-2 peptide pools (Spike and Membrane, Mem) and treated with Avasimibe (AVS) or DMSO for 8d. SARS-CoV-2-specific cytokine production by CD4+ T cells was detected via flow cytometry. The cytokine production/CD154 expression in wells without peptide stimulation was subtracted to determine SARS-CoV-2-specific cytokine production/CD154 expression in summary data. Example plots and summary data for SARS-CoV-2 specific IFNγ(a), TNF (b), MIP1β(c) production and CD154 expression (d) by CD4+ T cells (n=19). (e) Assessment of SARS-CoV-2-specific proliferation determined by CFSE dilution gated on IFNγ+ CD4+ T cells (Spike n=10; Mem n=11). Bars mean. Doughnut charts indicate fraction of donors with response to AVS (red). Response defined as de novo or increased cytokine production/CD154 expression. P values determined by Wilcoxon matched-pairs signed rank test.
Conclusions
Overall, the current study found that ACAT inhibition by AVS blocked SARS-CoV-2 fusion in VeroE6 and VeroE6-TMPRSS2 cells. The finding that AVS also inhibited VSV-G pseudo particles indicates its potential applicability against different viruses. AVS boosted CD4+ T-cells activated during acute SARS-CoV-2 infection and not virus-specific memory T-cells, even among the elderly, thus indicating that AVS could be tested as a vaccine adjuvant.
Overall, the researchers here demonstrated a unique dual functionality of AVS in inhibiting SARS-CoV-2 replication and boosting T-cell responses. These findings warrant the prioritization of future clinical trials of repurposed ACAT inhibitors such as AVS, given their excellent safety profile.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Schmidt, N. M., Wong, P. A. C., Peters, R., et al. (2022). An ACAT inhibitor regulates SARS-CoV-2 replication and antiviral T cell activity. bioRxiv. doi:10.1101/2022.04.12.487988. https://www.biorxiv.org/content/10.1101/2022.04.12.487988v1.
- Peer reviewed and published scientific report.
Wing, Peter AC, Nathalie M. Schmidt, Rory Peters, Maximilian Erdmann, Rachel Brown, Hao Wang, Leo Swadling, et al. 2023. “An ACAT Inhibitor Suppresses SARS-CoV-2 Replication and Boosts Antiviral T Cell Activity.” Edited by Stephanie Pfaender. PLOS Pathogens 19 (5): e1011323. https://doi.org/10.1371/journal.ppat.1011323. https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1011323.