To combat the COVID-19 pandemic, high-income countries acquired large quantities of vaccines. However, the storage and handling of vaccines are complex. Due to this, there have been massive losses in vaccine doses. In addition, there have been losses of doses in several African countries due to inadequate infrastructure. This has affected their population vaccination coverage.
According to the World Health Organization (WHO), vaccine wastage includes vaccines being discarded, lost, damaged, or destroyed. COVID-19 vaccines are available as multi-dose vials. Once opened, all doses have to be administered. The remaining doses cannot be stored and hence have to be discarded.
There are two reasons for vaccine wastage: discarding opened vials due to contamination while handling and discarding unopened vials due to reaching the date of expiry, higher storage temperature, or incorrect inventory. Thus, there should be strategies to improve vaccine handling to avoid vaccine wastage.
If opened vials could be re-frozen and utilized later, it would avoid vaccine wastage. However, we do not know if the re-freezing of vaccine vials affects their integrity and immunogenicity.
Testing the effects of re-freezing vaccines
This study assessed the consequences of re-freezing the remaining doses of COVID-19 mRNA vaccine vials after opening.
Two mRNA vaccines were tested in this study: Comirnaty (Pfizer/Biontech) and Spikevax (Moderna). The vaccine vials were stored and prepared for administration in syringes according to the manufacturer's instructions. These syringes were divided into three batches.
The first batch was administered immediately to mice. The second batch was frozen at -20°C for one month, defrosted, and then administered to mice. The third batch was frozen at −80°C for one month, defrosted, and then administered to mice. Along with injecting mice, a portion of the vaccine vial was tested for mRNA integrity. The mice were tested for behavioral and physiological changes post-vaccination to assess the possible occurrence of side effects. This included the Irwin test and test for locomotor activity. Irwin's test is a battery of tests that measures the behavioral parameters of a mouse in regularly spaced time intervals. The Irwin test was performed four times, at 24 h and 48 h post-vaccination and on days 2, 3, 22, and 23 for Comirnaty vaccine and on days 2, 3, 29, and 30 for Spikevax vaccine. The locomotor activity was measured using locomotor activity boxes at 24 h post-vaccination and on days 2 and 22 for the Comirnaty vaccine and on days 2 and 29 for the Spikevax vaccine.
Blood samples were collected 20 days after Comirnaty vaccination or 27 days after Spikevax vaccination and analyzed for antibodies against the receptor-binding domain (RBD) of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Spike protein.
Re-freezing did not affect vaccines
The mRNA integrity of the Comirnaty and Spikevax vaccines did not change after re-freezing the vials for one month at −20°C or −80°C. There was less than 5% of degradation of the Comirnaty vaccine mRNA and less than 6% of degradation of the Spikevax vaccine mRNA while administration of the vaccine doses to mice. However, these changes were not statistically significant.
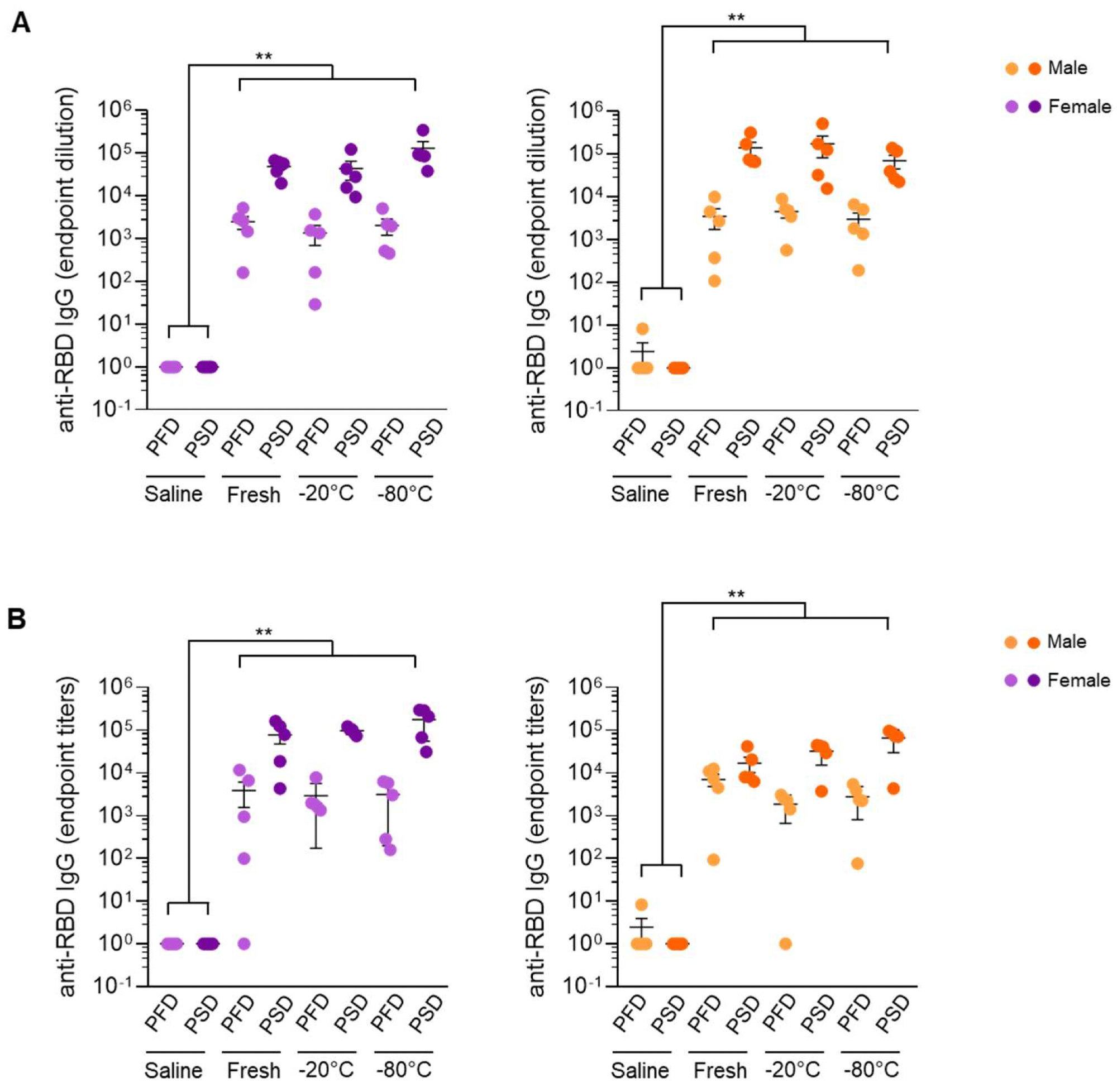 mRNA vaccines under different handling and re-freezing conditions induced similar responses in male and female mice. SARS-CoV-2 Spike RBD-specific IgG end-point titers of mice immunized with Comirnaty® (Pfizer-BioNTech) vaccine (A) or Spikevax® Moderna vaccine (B) post-prime and post-boost immunization. Sera from control mice inoculated with PBS were used to establish negative threshold values defined as the mean plus 4 times the standard deviation of the mean. Bars represent mean ± standard error mean (SEM). Dashed line indicates negative threshold. Data are presented as individual dots. Two-tailed Mann–Whitney U test was performed to compare vaccine treatment groups (saline, fresh, −20 °C, and −80 °C) (** p < 0.01). For each time point and condition, n = 5.
mRNA vaccines under different handling and re-freezing conditions induced similar responses in male and female mice. SARS-CoV-2 Spike RBD-specific IgG end-point titers of mice immunized with Comirnaty® (Pfizer-BioNTech) vaccine (A) or Spikevax® Moderna vaccine (B) post-prime and post-boost immunization. Sera from control mice inoculated with PBS were used to establish negative threshold values defined as the mean plus 4 times the standard deviation of the mean. Bars represent mean ± standard error mean (SEM). Dashed line indicates negative threshold. Data are presented as individual dots. Two-tailed Mann–Whitney U test was performed to compare vaccine treatment groups (saline, fresh, −20 °C, and −80 °C) (** p < 0.01). For each time point and condition, n = 5.
The administration of the first dose of both the mRNA vaccines induced the production of RBD-binding antibodies. The antibody levels further increased after the second dose of the vaccine. When the three groups of mice were compared, there were no significant differences in the antibody levels. Thus, the handling and storage conditions did not affect the immunogenicity of the vaccines.
Furthermore, the administration of both mRNA vaccines exposed to different handling and re-freezing conditions did not cause any side effects in mice after vaccination. There were no behavioral or physiological post-vaccination changes in mice.
Conclusion
Re-freezing mRNA vaccines did not affect mRNA integrity and immunogenicity. It also did not lead to any post-vaccination side effects. This study introduces the possibility of re-freezing the Comirnaty and Spikevax vaccine vials to limit vaccine wastage.
Implications of the study
Re-freezing mRNA vaccines in pre-filled syringes will help overcome the infrastructural inadequacies in middle- and low-income countries. It would also minimize vaccine wastage in high-income countries.
The observations made in this study could change the recommendations regarding the storage and handling of Comirnaty and Spikevax vaccines.