A recent research paper posted to the Research Square* preprint server and under review at Nature Portfolio journal assessed the dose fractionation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines.
 Study: Immunogenicity, efficacy, and safety of SARS-CoV-2 vaccine dose fractionation: a systematic review and meta-analysis. Image Credit: siam.pukkato / Shutterstock
Study: Immunogenicity, efficacy, and safety of SARS-CoV-2 vaccine dose fractionation: a systematic review and meta-analysis. Image Credit: siam.pukkato / Shutterstock

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
Coronavirus disease 2019 (COVID-19) continues to be a global health challenge three years after the pandemic began, with the subsequent emergence of novel SARS-CoV-2 variants. While SARS-CoV-2 vaccinations have saved tens of thousands of lives and lowered hospitalizations in high-income nations, as of April 1, 2022, only 14.5% of citizens in low-income countries have received at least one vaccine dose. Dosage fractionation of vaccines has previously been proved (yellow fever, 2016) to alleviate global supply shortages and expedite vaccine coverage in low-income nations. Therefore, a higher proportion of the community could be vaccinated though receiving a lower vaccine dose per person via this approach.
Hence, dose fractionation of the COVID-19 vaccine might substantially speed up universal vaccine coverage. Yet, concerns and uncertainties about the effectiveness of fractional dose vaccinations against SARS-CoV-2 ancestor sequences and emerging variants of concern (VOCs), and possible discrepancies among vaccine platforms, hampered the endorsement of COVID-19 vaccines dose fractionation. Thus, supporting data on the effectiveness, safety, and immunogenicity of fractionated COVID-19 vaccine dose is required, particularly in the context of emerging SARS-CoV-2 mutants.
About the study
In the present study, the scientists evaluated the efficacy, safety, and immunogenicity of COVID-19 vaccine dose fractionation. They conducted a meta-analysis and systematic review of phase 2/3 studies that published dose-finding data on COVID-19 vaccines safety and immunogenicity to assess the dose-response association of SARS-CoV-2 vaccines-induced neutralizing antibodies (nAbs).
The scientists also used previously published indicators of cross-reactivity and protection conferred by nAbs to predict fractional dose vaccine effectiveness against both SARS-CoV-2 ancestral and mutated strains. Further, the team evaluated the safety profiles and seroconversion of nAbs and T-cell responses between standard and fractional-dose groups. This was to analyze the variations in safety and immunogenicity post-standard and fractional doses.
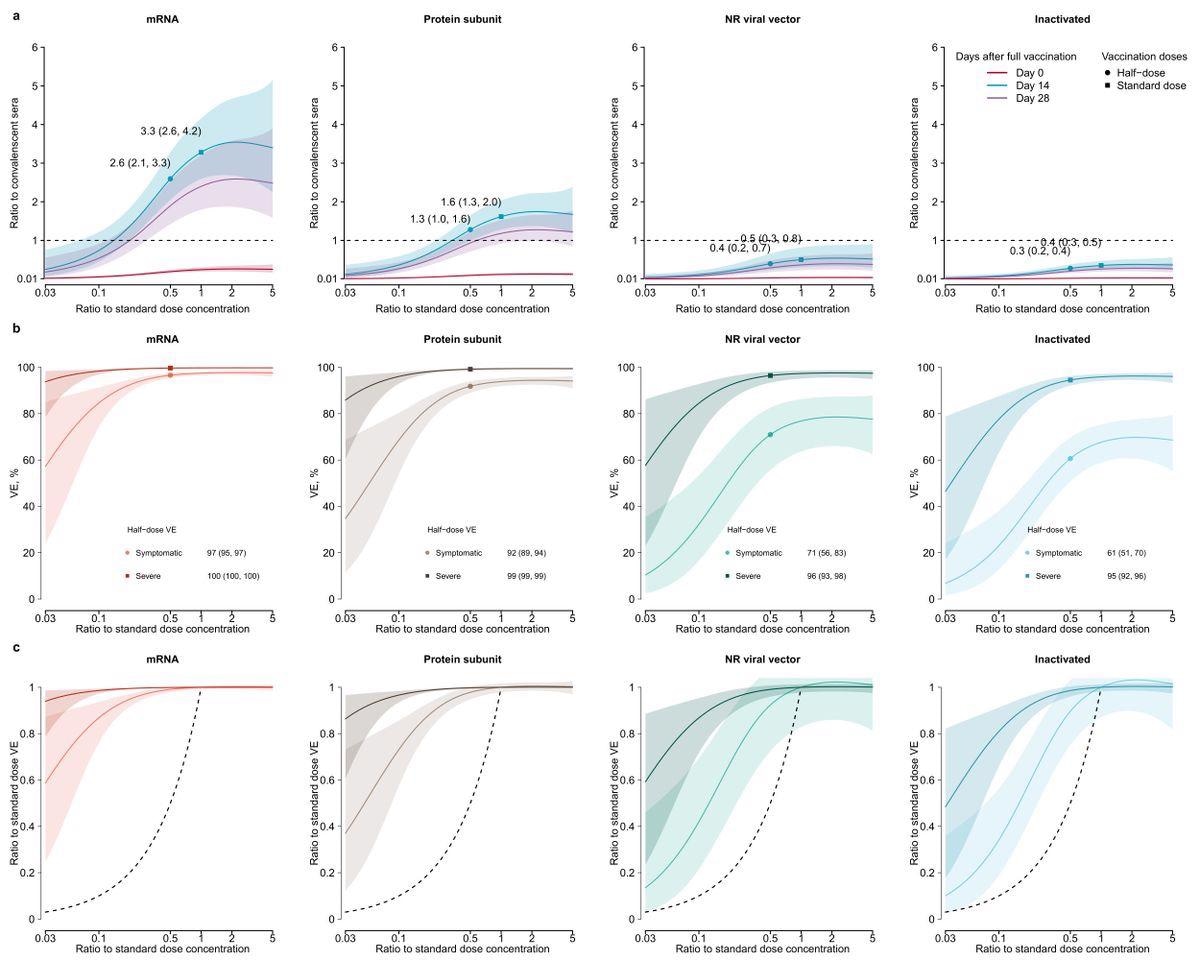
Dose-response relationship of neutralizing antibodies (nAbs) and vaccine efficacy (VE) against ancestral strains induced by COVID-19 vaccines. (a) Dose-response relationship of nAbs against ancestral strains. nAbs were standardized as the ratio to the convalescent sera. A 2-dose schedule was assumed for RNA, protein subunit and inactivated vaccines, while 1-dose schedule was assumed for non-replicating viral vector. Dashed horizontal line indicates the average level of nAbs against ancestral strains in convalescent sera. (b) Dose-response relationship of predicted vaccine efficacy against symptomatic and severe infections of ancestral strains. (c) Association between reduction in vaccine efficacy and dose fractionation. Reductions in vaccine efficacy were measured as the ratio between vaccine efficacy against symptomatic or severe infections of ancestral strains between fractional and standard-dose groups.
Results and discussions
According to the results, vaccination-induced nAbs against SARS-CoV-2 differed significantly among vaccine platforms and dose fractions. Standard doses in SARS-CoV-2 protein subunit and messenger ribonucleic acid (mRNA) vaccine platforms could induce significant nAbs than COVID-19 recovered sera. In addition, the authors found that dosage fractionation might elicit considerable nAbs towards the SARS-CoV-2 original strains and equivalent seroconversion percentage with the standard doses.
Except for symptomatic Omicron and Beta infections, nAbs generated by fractional subunit and mRNA vaccines were projected to impart ≥ 65% effectiveness against severe and symptomatic SARS-CoV-2 infections. Fractional dose-induced detectable nAbs might impart > 50% protection against symptomatic SARS-CoV-2 infection with ancestral strains according to previously established correlates of protection (CoP).
Except for the mRNA-1273 vaccine, the fractionation of vaccination doses appeared safe and elicited strong type 1 T helper (Th1) biased T-cell responses comparable to conventional doses. Fractional doses of most vaccinations might elicit sensitive and presumably robust T-cell responses, perhaps enhancing vaccine protection against catastrophic consequences. SARS-CoV-2-specific T-cells exhibit a broad cross-reactivity to numerous VOCs, including Omicron, and were linked to improved outcomes. Hence, even during the emergence of the new SARS-CoV-2 variants with vaccine breakthrough infections, COVID-19 vaccine dosage fractionation might still lower hospitalizations and fatalities significantly.
The authors found that nAbs, and hence vaccination effectiveness, were heightened in two half-doses than in a single conventional dose. By splitting one dosage into two, it may be possible to avoid more hospitalizations and fatalities due to the anticipated fractional dose-elicited substantial protection against severe infections by both mutant and ancestral strains. Shipping standard dosages and dividing at vaccine campaign locations may make dose fractionation more affordable by decreasing logistical expenses, such as costs linked with manufacturing and transportation.
The team could not analyze the longevity of the immune responses generated by fractional doses of SARS-CoV-2 vaccinations since most trials only had a one-month follow-up period. As evidence from regular dose vaccinees after six months suggests, a decline in SARS-CoV-2 specific T-cells, Abs, and vaccine effectiveness against both VOCs and ancestor strains could be predicted for fractional doses. Both heterogeneous and homogeneous boosters might significantly improve vaccine effectiveness and nAbs against VOCs for conventional doses, whereas evidence on fractional dosages was limited.
Due to a lack of data, the scientists did not investigate the nAbs elicited by particular vaccine manufacturers but found a consistent seroconversion percentage and dose association inside the platform. Despite this, various vaccinations from the same platform exhibited variations in nAbs and longevity, such as BNT162b1 versus mRNA-1273 vaccines.
Conclusions
The study findings revealed that dose fractionation of COVID-19 protein subunit and mRNA vaccines might generate nAbs and T cells specific towards SARS-CoV-2 original and mutant sequences that possibly impart considerable protection with vaccine effectiveness above 50%. The team found that fractional dosages have similar safety characteristics to the standard vaccine dose. Further, the present data suggested that the fractional dose approach might lead to improved use of the limited SARS-CoV-2 antigen stock.
Overall, the present work indicated that SARS-CoV-2 protein subunit and mRNA vaccines could be dose fractionated safely and effectively.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Bingyi Yang, Xiaotong Huang, Huizhi Gao et al. Immunogenicity, efficacy, and safety of SARS-CoV-2 vaccine dose fractionation: a systematic review and meta-analysis, April 28 2022, PREPRINT (Version 1) available at Research Square [https://doi.org/10.21203/rs.3.rs-1571821/v1], https://www.researchsquare.com/article/rs-1571821/v1
- Peer reviewed and published scientific report.
Yang, Bingyi, Xiaotong Huang, Huizhi Gao, Nancy H. Leung, Tim K. Tsang, and Benjamin J. Cowling. 2022. “Immunogenicity, Efficacy, and Safety of SARS-CoV-2 Vaccine Dose Fractionation: A Systematic Review and Meta-Analysis.” BMC Medicine 20 (1). https://doi.org/10.1186/s12916-022-02600-0. https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-022-02600-0.