mRNA-1273 encodes for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike (S-2P) protein of the prototype Wuhan-Hu-1 (WA1) isolate and induces robust neutralizing antibody (nAb) responses to WA1 and the D614G variant.
Compared to the D614G strain, mRNA-1273 induced weaker nAb responses against SARS-CoV-2 variants Beta and Delta, particularly for Beta. Therefore, an mRNA-1273.351 vaccine variant encoding Beta S-2P was developed. However, hospitalizations and breakthrough infections are still occurring in vaccinated individuals, thus, necessitating the determination of the optimal third dose for all three vaccine formulations.
 Study: Safety and Immunogenicity of a Third Dose of SARS-CoV-2 mRNA Vaccine — An Interim Analysis. Image Credit: Numstocker / Shutterstock
Study: Safety and Immunogenicity of a Third Dose of SARS-CoV-2 mRNA Vaccine — An Interim Analysis. Image Credit: Numstocker / Shutterstock

 *Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
About the study
In the present study, researchers evaluated three vaccine formulations of the mRNA-1273 vaccine:
i) a booster dose of 100 mcg mRNA-1273 (monovalent prototype),
ii) a booster dose of 50 mcg of mRNA-1273.351 (monovalent variant), and
iii) a booster dose of 25 mcg of mRNA-1273 and mRNA-1273.351 each (bivalent).
The evaluation was part of the phase I dose-finding trial of mRNA-1273 that took place between March and June 2020. They administered the booster doses of mRNA-1273 between March and April 2021 and followed up with the recipients for 28 days. However, follow-up continued for 12 months to assess vaccine safety, reactogenicity, and immunogenicity.
The researchers stratified the study participants into three age-based groups, 18-55, 56-70, and over 71 years. Then, the team administered the vaccine formulations eight to 12 months after the study participants had received a two-dose mRNA-1273 primary series at doses of 250, 100, 50, or 25 mcg, within a gap of 28 days.
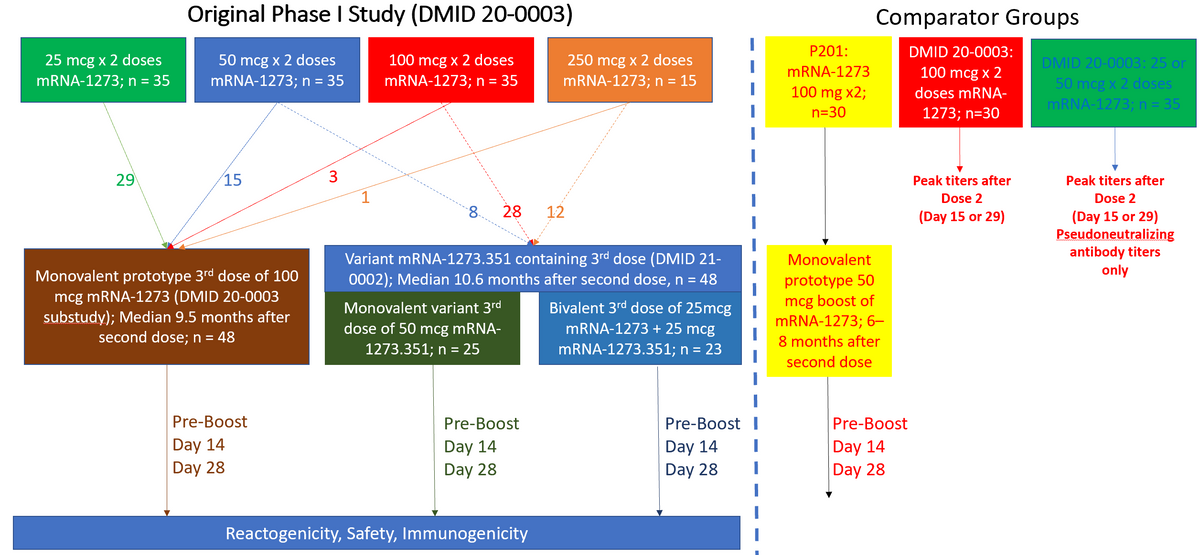
Enrollment and Timing of Samples Obtained for Participants and Comparators in this Study. The original Phase I mRNA-1273 study enrolled subjects ≥18 years of age and administered two doses of mRNA-1273 across 4 different dose levels (25, 50, 100, and 250 mcg). Those that had received any dose level were allowed to receive a single 100 mcg third dose (monovalent prototype vaccine group). Those that had received 50, 100, or 250 mcg doses were allowed to join a new study in which they were randomized to a single 50 mcg dose of monovalent variant vaccine (mRNA-1273.351) or a single dose of bivalent vaccine (25 mcg of mRNA-1273, 25 mcg mRNA-1273.351). For comparison, 30 adults that had received an initial 100 mcg of mRNA-1273 in the Phase 2 study followed by a 50 mcg third dose were included (P201). Peak responses observed at 2 or 4 weeks after a second dose of mRNA-1273 in those that had enrolled in the original Phase I study were used for comparison.
The researchers evaluated the monovalent prototype booster vaccine among 48 participants, who tended to be older and had received their booster dose at a median of 9.5 months after the second dose.
There were 48 participants in the bivalent and monovalent variant groups, of which 12, 28, and 8 had received 250, 100, or 50 mcg doses of mRNA-1273, respectively. They received their third dose at a median of 10.6 months after the second vaccination.
The team tested sera obtained before booster vaccination, on the day of booster vaccination, through 14 days after the booster. They used a Mesoscale Discovery (MSD) 10-plex assay to detect SARS-CoV-2 binding immunoglobulin G (IgG) antibodies.
They used pseudovirus neutralization assay (PsVN) to determine serum inhibitory dilution required to achieve 50% and 80% viral neutralization, ID50 and ID80, respectively. They also performed a focus-reduction neutralization test (FRNT) assay to evaluate vaccine-induced neutralizing activity against the D614G, Beta, and other SARS-CoV-2 variants.
Lastly, they used 27-color flow cytometry to examine the SARS-CoV-2-specific cluster of differentiation 4 (CD4)+ and CD8+ T-cell responses.
Study findings
The participants who received 25 or 50 mcg doses of mRNA-1273 showed waning immunity. Therefore, the researchers amended the protocol and offered a booster dose of 100mcg mRNA-1273 to the participants of the monovalent prototype group. Similarly, they offered a booster dose of 100mcg mRNA-1273 to other high-dose (50, 100, or 250 mcg) recipients in the monovalent variant and bivalent groups.
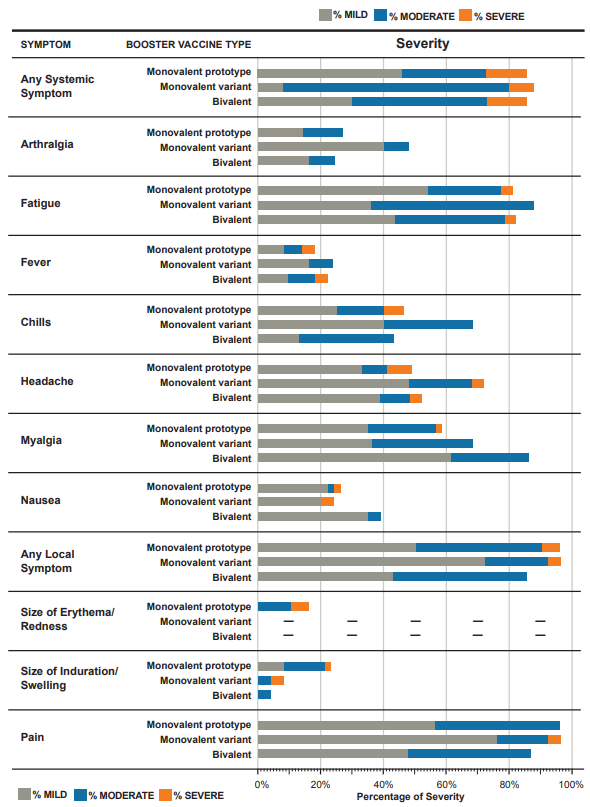
Reactogenicity in Participants After a Third Dose by Group. Solicited adverse events in the week after receiving a third dose of mRNA vaccine by study group: Monovalent prototype (50 mcg of mRNA-1273), Monovalent variant (50 mcg of mRNA-1273.351), and Bivalent (25 mcg of mRNA-1273, 25 mcg of mRNA-1273.351). Reactogenicity was generally similar across the study groups. Erythema/redness was not observed by participants in the monovalent variant and bivalent groups.
Across the three booster vaccine recipient groups, the type and frequency of adverse reactions after the third dose of mRNA vaccine were similar. Most adverse reactions were mild, and severe reactions occurred only in 8 – 13% of participants across the three groups.
Regardless of age and initial primary series dose, the binding antibodies and nAbs continued to decline in the booster recipients but remained detectable up to 11 months before the third dose. Conversely, pre-boost neutralization titers against Delta and Beta variants declined substantially after the 100 mcg first dose. Notably, post-booster dose, nAb titers exceeded levels attained 14 days after the second vaccine dose against each tested variant.
In individuals who had received 25 or 50 mcg doses of the primary vaccine series, nAb responses had waned by the time of the booster dose. The third dose of vaccine resulted in geometric mean titers (GMTs) of over 500 for all variants, translating to over 90% vaccine efficacy.
Although T effector and central memory CD4+ T cell responses were most robust after the third dose, T helper cell type 1 (TH1)-biased CD4+ responses were in the same proportions as those observed after the second dose across all three studies groups. The authors also noted S-specific CD8+ T cells responses in 40 to 48% of recipients of the booster dose.
Conclusions
After the primary mRNA-1273 vaccine series, humoral and cellular immune responses persisted for 10 to 11 months. The booster dose enhanced levels of binding antibodies that recognized the S proteins of all the tested variants, including Beta and Delta. Booster doses of all three vaccine formulations - monovalent prototype, monovalent variant, and bivalent - resulted in functional nAb responses against all the tested SARS-CoV-2 variants.
Taken together, these findings support the use of a monovalent mRNA-1273 booster for extensive immunological cross-protection across several SARS-CoV-2 variants. However, novel multivalent mRNA vaccines must also be considered for development to improve cross-immune protection in the long term.

 *Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Evan Anderson, Lisa Jackson, Nadine Rouphael, Alicia Widge, David Montefiori, Nicole Doria-Rose, Mehul Suthar, Kristen Cohen, Sarah O'Connell, Mat Makowski, Mamodikoe Makhene, Wendy Buchanan, Paul Spearman, C. Buddy Creech, Sijy O'Dell, Stephen Schmidt, Brett Leav, Hamilton Bennett, Rolando Pajon, Christine Posavad, John Hural, John Beigel, Jim Albert, Kuleni Abebe, Amanda Eaton, Christina Rostad, Paulina Rebolledo, Satoshi Kamidani, Daniel Graciaa, Rhea Coler, Adrian McDermott, Julie Ledgerwood, John Mascola, Stephen DeRosa, Kathleen Neuzil, M. Juliana McElrath, Paul Roberts, Safety and Immunogenicity of a Third Dose of SARS-CoV-2 mRNA Vaccine — An Interim Analysis, Research Square preprint 2022, DOI: https://doi.org/10.21203/rs.3.rs-1222037/v1, https://www.researchsquare.com/article/rs-1222037/v1