Many novel designs and drug delivery systems have emerged over the past few years, allowing scientists to envisage the application of medicine based on ribonucleic acid (RNA) in the treatment, prevention, and diagnosis of an array of disorders. The development of messenger RNA (mRNA) vaccines against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has only increased the impetus in this field.
This is “the largest human-curated collection of published scientific knowledge, used for quantitative analysis of global scientific publications.”
 Study: The Progress and Promise of RNA Medicine─An Arsenal of Targeted Treatment. Image Credit: MattLphotography / Shutterstock
Study: The Progress and Promise of RNA Medicine─An Arsenal of Targeted Treatment. Image Credit: MattLphotography / Shutterstock
Introduction
RNA is among the most important biomolecules, encoding genetic information as mRNA, or existing as noncoding RNA (ncRNA). The latter include ribosomal RNA (rRNA), transfer RNA (tRNA), small nuclear RNA (snRNA), long ncRNA, small nucleolar RNA (snoRNA), and short hairpin RNA (shRNA), and the like. The first three are the most abundant, making up 1-5%, 80%, and 15% of the RNA content of an active cell.
The first, that is, mRNA, exists a molecule with a 5’-7-methylguanosine cap, a 5’ untranslated region (5’-UTR), and a 3’-polyadenosine (polyA) tail. The balance between mRNA synthesis and breakdown determines gene expression levels.
Again, siRNAs come from lncRNAs and are key to RNAi and thus to the inhibition of gene expression. These molecules come from double-stranded RNA (dsRNA) that is cut by the Dicer endonuclease into up to 24 base-pair sections overhanging by a little on either end. These are the siRNAs that form the pre-RNA-induced silencing complex (pre-RISC) along with the Argonaute protein. The recognition of complementary mRNA to the siRNA is followed by endonuclease-mediated cutting of the targeted mRNA and reduced gene expression. Another mechanism by which RISC reduces gene expression is by blocking ribosome binding and translation.
Many important events litter the landscape of RNA medicine, from the discovery of mRNA in the 1960s to the 5’-cap on mRNA in the next ten years. Cell delivery systems such as RNA encapsulated in liposomes and in vitro transcription in cell-free systems using bacteriophage and RNA polymerase elements followed in the next decade.
Later on, mRNA delivery by cationic lipids was reported, followed by the first of the antisense RNA drugs late in the 90s, along with the discovery of RNA interference (RNAi). These were foundational to the production of RNA therapeutic drugs. SiRNA was the first siRNA-based drug approved in 2018, while the CRISPR-Cas9 gene-editing system won the Nobel Prize for the scientists who developed it.
Finally, the first mRNA COVID-19 vaccines received full approval in 2021. However, over the past seven years, a number of RNA therapeutic treatments were approved for conditions like Duchenne muscular dystrophy, amyotrophic lateral sclerosis, and macular degeneration. Many others are in the pipeline.
However, RNA is a fragile biomolecule, easily and rapidly broken down, and might be immunogenic. Adaptations by chemical modification of the RNA molecule are one way out of this difficulty, involving the base, backbone, sugar, and 5’ and 3’ ligations to other molecules. Encapsulating RNA within nanoparticles for intracellular delivery is another method to protect the RNA from degradation outside the cell.
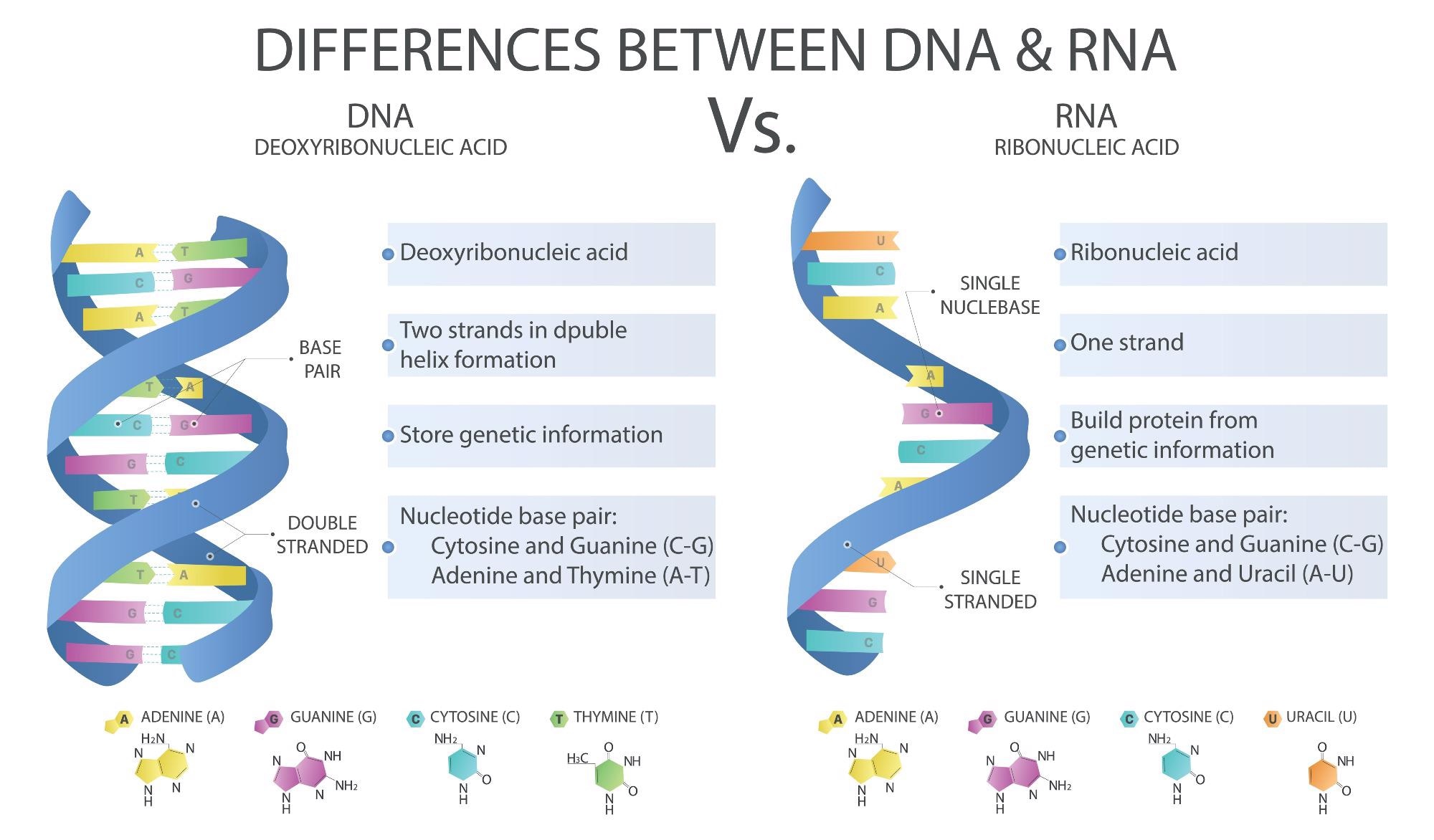 Difference between DNA and RNA. Image Credit: DiBtv / Shutterstock
Difference between DNA and RNA. Image Credit: DiBtv / Shutterstock
Types of Medically Used RNA
Many different types of RNA are used therapeutically or diagnostically. An mRNA may be translated into a functional protein that is naturally lacking in the target cell. MicroRNAs (miRNAs) that mark mRNAs for breakdown by binding to their 3’-UTR, serving as regulatory checkpoints and biomarkers for disease diagnosis.
Conversely, short interfering RNAs (siRNAs) bind to specific points in the mRNA coding region, making them attractive as target-specific drugs, but often with off-target effects as well that are serious disadvantages. Increased target specificity is achieved by reducing stable binding between the siRNA and non-target mRNAs, by introducing groups that cause steric hindrance, like e 2′-O-methyl and 2′-MOE ribose modifications.
Others include antisense oligonucleotides (ASOs) and antibody-oligonucleotide conjugates (AOCs), which detect and bind complementary sequences in DNA or RNA to overcome mutated sequences via RNA splicing or non-translation of a non-functional protein. RNAs are used as guides in a CRISPR-Cas system to target the specific cleavage site in the genome.
There are several advantages of using RNAs in therapeutic approaches, including their specificity, modularity, customizability, predictability and inexpensiveness, as well as their inability to interfere with the genome, which makes them safe. They are also easy to synthesize on a commercial scale.
“Thus, the development of RNA therapeutics and vaccines is relatively fast and straightforward. This was demonstrated by the development, testing, and administration of the COVID-19 mRNA vaccines within a year of the isolation and sequencing of the SARS-CoV-2 viral genome.”
Disadvantages include easy breakdown, off-target effects, and unknown efficacy.
Types of RNA Being Developed Currently
Research on RNA medicine comes from a number of fields, with the majority coming from the field of cancer, as well as liver, lung, and metabolic diseases. Many patents for RNA medicine come from the USA or China.
Some companies that specialize in mRNA therapeutics include Moderna, BioNTech, and Stemirna Therapeutics. The first has over 45 RNA therapeutics in the development process, while over 130 are being developed for therapeutic or diagnostic patents. Others use siRNAs such as Sirnaomics, Alnylam, and Arrowhead Therapeutics, or ASOs such as Ionis Pharmaceuticals or Sarepta Therapeutics.
Interestingly, except for two, the top 15 companies focus on a single type of RNA. The exceptions are AstraZeneca and CureVac. Again, all but two targets a single disease condition.
Many RNA-based drugs are in the pipeline for cardiovascular disease, metabolic disease, and cancers. Infectious diseases are another primary target, such as the COVID-19 mRNA vaccines, RNAi drugs meant to reduce immune evasion of host responses by viruses like the hepatitis B virus, and Moderna’s quadrivalent seasonal influenza mRNA vaccine candidate.
Chemical Modifications
Multiple modifications have been made to RNA structure in order to protect the backbone from attack by nucleases while reducing off-target effects. These include modifications of the bases by methylation, oxygen replacement by sulfur, or of ring nitrogen with carbon; ribose modifications; and backbone modifications to resist digestion by nucleases and promote the entry of RNA into the cell through the nonpolar lipid bilayer.
It is easier to add modified nucleotides to a baseline RNA to create small artificial RNAs, while larger RNAs are generated by in vitro or in vivo transcription, and are less easily modified because of the limitations of enzymatic activity. Besides, modification to a large extent may produce steric hindrance, preventing proper translation.
Different modifications are used to a larger extent for specific types of RNA, to enhance their effectiveness. Backbone modifications can be tuned to block specific cell processes or target an RNA for degradation by nucleases, while ribose modifications can be used with siRNAs to reduce off-target effects by reducing the thermal stability. Uridine is replaced with 1-methylpseudouridine in therapeutic mRNAs to reduce their immunogenicity and increase translation.
ASOs and siRNAs meant for therapeutic use are engineered with phosphorothioate and 2′-ribose modifications to protect against degradation and prevent off-target effects.
RNA Delivery Systems
Finally, many researchers have presented novel methods of RNA delivery into the cell. the hydrophilic anionic therapeutic RNA molecules are unable to cross the cell membrane without delivery systems or being chemically modified. Systemic administration necessitates the use of a protective delivery vehicle against degradation, evades immune surveillance, does not interact with serum proteins, and hinders the excretion of the drug through the kidneys.
Nanomaterials that are biodegradable, biocompatible, and have low toxicity are being used today as nanocarriers of RNA. These include lipids polyethylenimine (PEI), poly(lactic-coglycolic acid), magnetic nanoparticles, carbon nanotubes, gold nanoparticles, and silica nanoparticles. Lipid nanoparticles (LNPs) are currently used in nucleic acid vaccines and drugs due to their easy manufacture and meeting the above criteria.
Liposomes represent the first generation of LNPs. They are also immunological adjuvants and have been used in many COVID-19 vaccines. Exosomes are natural counterparts of the liposomes, but with some advantages such as lower toxicity, less immunogenicity, and the ability to cross natural barriers like the blood-brain barrier. They also do not cause the drug to build up in the liver.
Red blood cells are another promising natural carrier, lacking nuclei and mitochondrial DNA, thus avoiding the risk of integration of the therapeutic RNA into the host genome. Additionally, macrophages are being studied in order to treat solid cancers since they are found in tumors.
Other vehicles include polymeric NPs, gold NPs, and mesoporous silica nanoparticles (MSNs). The latter are non-toxic and degrade slowly, allowing slow release of the drug over a long time. They also have large surfaces, with adaptable pore sizes, and have the ability to incorporate drug molecules efficiently.
Ligand-conjugated RNA NPs are another avenue, relying on the assembly of RNA into complex structures that can be modified as per the requirement. Origami-like RNA nanostructures are also promising vehicles, as are virus-like particles (VLPs).
Conclusion
RNA's uses and categories have been better understood over the past half-century, and this knowledge has been utilized to develop improved therapeutics. It is helpful to have a variety of different types of RNA to regulate specific cell processes. As RNA carriers develop, the use of novel vehicles, including carbon nanodots and nanotubes, and functionalized systems is growing.
“Our growing understanding of the many types and functions of RNA has been combined with the ability to synthesize modified RNAs with improved stability and pharmaceutical activity. RNA therapeutics have the potential to treat a wide range of diseases, from the most common to the extremely rare.”