Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections cause mild or no symptoms in a few individuals, whereas severe pulmonary injury or death in the others. Due to such varied clinical presentation, identifying biomarkers of COVID-19 severity would highly benefit COVID-19 patients is essential.
Factors necessary for SARS-CoV-2 invasion into the host include transmembrane serine protease 2 and 4 (TMPRSS 2, 4) and angiotensin-converting enzyme 2 (ACE2) are expressed in high amounts in salivary glands and cells of the oral epithelium. Furthermore, the microbiome of the nasopharynx bears further exploration due to the fact that the mucosa of the nasopharynx is the primary site of infection, multiplication, and dissemination of SARS-CoV-2 in host tissues and organs.
About the study
In the present study, researchers explored the role of salivary and nasopharyngeal microbiomes in the severity of SARS-CoV-2 infections.
Saliva (oral) samples and nasopharyngeal swab samples were obtained from 60 and 54 SARS-CoV-2-positive patients, respectively, who were symptomatic, and their COVID-19 diagnosis was confirmed by polymerase chain reaction (PCR). Most of the samples were obtained within two weeks of COVID-19 symptom onset. For comparison, 18 and 12 salivary and nasopharyngeal swab samples were obtained from SARS-CoV-2-negative individuals, respectively.
Microbial deoxyribonucleic acid (DNA) extracted from the samples was subjected to 16S ribosomal ribonucleic acid (RNA) gene sequencing for microbiome characterization. Additionally, the association between nasopharyngeal and salivary microbiomes, plasma cytokine levels, and age was assessed.
The association between community-level alterations in oral and nasopharyngeal microbiomes and COVID-19 symptoms was assessed by comparing the alpha diversity and beta diversity among symptomatic COVID-19 and non-COVID-19 participants. COVID-19 symptoms assessed were fever, shortness of breath, coughing, nausea/vomiting, and diarrhea. COVID-19 severity was determined based on the status of intensive care unit (ICU) admissions.
Furthermore, differential abundance analysis was performed for the salivary and nasopharyngeal microbiomes of ICU, and non-ICU COVID-19 patients for genus level assessment of microbial differences at the taxa level among patients with severe COVID-19 and non-severe COVID-19. The correlation between microbial findings and systemic immune responses was determined based on the associations between the relative abundances of each genus and plasma cytokine levels.
Results
In the gene sequencing analysis, for oral samples and nasopharyngeal swab samples, the average reads per participant were 17,820 and 8,543, respectively. The five phyla most abundant in the oral microbiome were Actinobacteria, Firmicutes, Bacteroidetes, Fusobacteria, and Proteobacteria. SARS-CoV-2-positive patients demonstrated a lower abundance of Fusobacteria and Proteobacteria in the oral microbiome.
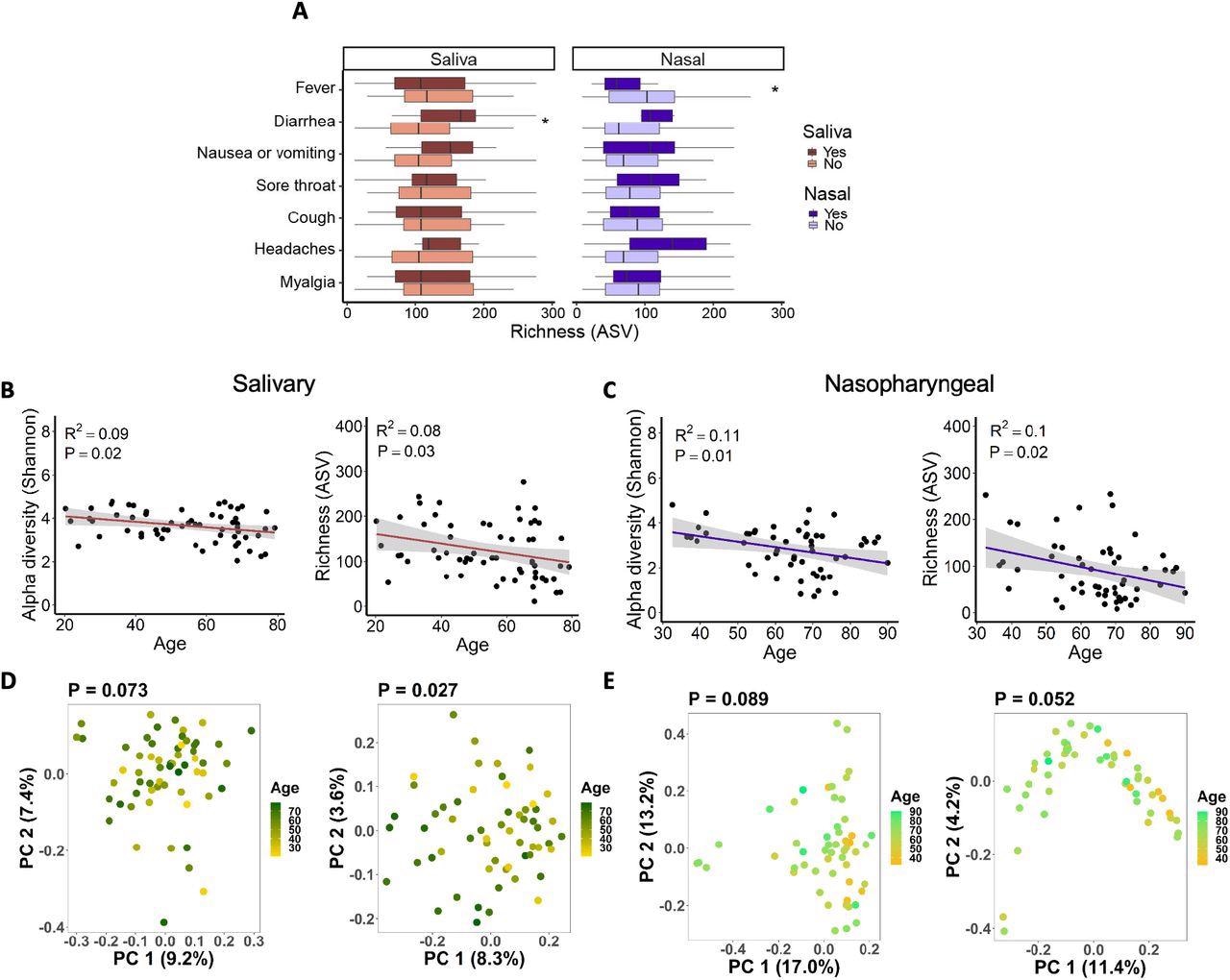
Associations between salivary and nasopharyngeal microbiomes of SARS-CoV-2-positive patients and COVID-19 symptoms and age. (A) Alpha diversity, represented by richness, in saliva (red) and nasopharyngeal swab (blue) samples, for patients with a given symptom (dark red or dark blue) or without (light red or light blue). Age versus alpha diversity represented by Shannon index and richness of salivary (B) and nasopharyngeal (C) microbial communities. Principal coordinates (PC) analysis of weighted (D, E, left) and unweighted (D, E, right) UniFrac distances for salivary (D) and nasopharyngeal (E) microbial communities with age. The shaded areas in panels B and C indicate the 95% confidence intervals. Statistical significance was assessed using the Wilcoxon signed-rank test for panel A, linear regression for panels B and C, and PERMANOVA for panels D and E. *, P < 0.05.
The top five phyla in the microbiome of the nasopharynx were Actinobacteria, Firmicutes, Fusobacteria, Proteobacteria, and Bacteroidetes, and their abundances did not significantly differ between COVID-19 patients and non-COVID-19 patients. In comparison to non-severe COVID-19 patients, oral Bifidobacterium, Solobacterium, and Lactobacillus were depleted in patients with severe COVID-19. In addition, nasopharyngeal microbes such as Proteus, Cupravidus, and Lactobacillus were increased, whereas Paracoccus was depleted in patients with severe COVID-19.
The abundance of Bifidobacterium in the salivary microbiome was negatively associated with plasma levels of COVID-19 biomarkers such as monocyte chemoattractant protein-1 (MCP-1) and interleukin 17F (IL-17F). SARS-CoV-2 infection status but not the infection severity was associated with community-level alterations in the oral and nasopharyngeal microbiomes.
Alpha diversity of oral and nasopharyngeal microbiomes was substantially decreased among COVID-19 patients compared to non-COVID-19 patients. However, no significant differences were observed in alpha diversity between ICU and non-ICU patients for the salivary and nasopharyngeal microbiomes. Salivary and nasopharyngeal microbiome alpha diversity demonstrated a negative correlation with age and were associated with diarrhea and fever.
In saliva samples, Bifidobacterium, Lactobacillus, and Solobacterium were more abundant in the non-ICU patients. A greater abundance of oral Bifidobacterium was associated with lower MCP-1 and IL-17F levels. In nasopharyngeal samples, Proteus, Cupravidus, and Lactobacillus were more abundant in the ICU group, whereas Paracoccus was more abundant in the non-ICU group.
To summarize, the study findings demonstrated the oral and nasopharyngeal microbial profiles of COVID-19 patients and showed that COVID-19 severity was associated with the relative abundance of certain bacterial taxa.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
The salivary and nasopharyngeal microbiomes are associated with SARS-CoV-2 infection and disease severity. Josh G. Kim, Ai Zhang, Adriana M. Rauseo, Charles W. Goss, Philip A. Mudd, Jane A. O'Halloran, Leyao Wang. bioRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.05.31.494162, https://www.biorxiv.org/content/10.1101/2022.05.31.494162v1
- Peer reviewed and published scientific report.
Kim, Josh G., Ai Zhang, Adriana M. Rauseo, Charles W. Goss, Philip A. Mudd, Jane A. O’Halloran, and Leyao Wang. 2022. “The Salivary and Nasopharyngeal Microbiomes Are Associated with SARS-CoV-2 Infection and Disease Severity.” Journal of Medical Virology, December. https://doi.org/10.1002/jmv.28445. https://onlinelibrary.wiley.com/doi/10.1002/jmv.28445.