New research reveals that milk fosters beneficial gut bacteria like Faecalibacterium and Akkermansia, while cheese reduces certain microbes - reshaping how dairy impacts digestive health.
 Study: Dairy Consumption and the Colonic Mucosa-Associated Gut Microbiota in Humans - A Preliminary Investigation. Image Credit: New Africa / Shutterstock
Study: Dairy Consumption and the Colonic Mucosa-Associated Gut Microbiota in Humans - A Preliminary Investigation. Image Credit: New Africa / Shutterstock
In a recent study published in the journal Nutrients, researchers in the United States explored the influence of dairy consumption on colonic mucosa-associated gut microbiota. By investigating specific bacterial composition changes linked to dairy intake, they highlighted its implications for individual and public health.
Background
Did you know that the human gut houses trillions of bacteria that influence everything from digestion to mental health? Research increasingly points to diet as a crucial factor in shaping our gut microbiome, yet the role of dairy remains controversial. While dairy provides essential nutrients such as calcium, vitamins, and probiotics, conflicting studies raise concerns about its effects on gut health. Some research links dairy consumption to enhanced beneficial gut bacteria, while others suggest potential risks such as inflammation and metabolic disturbances. Given the global prevalence of dairy consumption, understanding its precise effects on gut microbiota is critical for shaping dietary guidelines and public health initiatives. Further research is needed to determine how specific dairy products affect different bacterial species and their long-term influence on health.
About the study
A cross-sectional study was conducted with 34 participants who had undergone a colonoscopy at the Michael E. DeBakey Veterans Affairs Medical Center in Houston, Texas. Participants were selected based on strict eligibility criteria, excluding individuals with inflammatory bowel disease (IBD), recent antibiotic use, or major dietary changes. Self-reported dairy intake over the past year was assessed using a validated food frequency questionnaire (FFQ). Nutrient intake was adjusted for caloric consumption.
Colonic mucosal biopsies were collected and analyzed for microbial composition using 16S ribosomal Ribonucleic acid (rRNA) gene sequencing. Bacterial Deoxyribonucleic Acid (DNA) was extracted, and the V4 region of the 16S rRNA gene was amplified and sequenced using the Illumina MiSeq platform. Operational Taxonomic Unit (OTU) classification was performed using the Unified Platform for Automated Sequence Analysis (UPARSE) and structured Identification of Lifeforms from Various Environments databases (SILVA). Alpha-diversity (species richness and evenness) and beta-diversity (community composition differences) were calculated. Statistical analyses included negative binomial regression models adjusted for demographic and lifestyle factors such as age, body mass index (BMI), smoking status, alcohol use, and dietary quality. The study included a total of 97 mucosal biopsies from these participants. False discovery rate (FDR)-adjusted p-values were used to determine statistical significance.
Study results
Higher consumption of total dairy and milk was associated with increased microbial alpha-diversity, indicating greater bacterial richness and evenness. In contrast, higher cheese consumption was linked to lower microbial diversity. Beta-diversity analysis revealed significant differences in gut bacterial composition based on dairy intake levels.
Participants who consumed more dairy and milk exhibited a higher relative abundance of Faecalibacterium, a bacterium known for its anti-inflammatory properties. Increased milk intake was also associated with greater levels of Akkermansia, a mucin-degrading bacterium linked to improved gut barrier function and metabolic health. However, the association between Akkermansia and milk intake was attenuated after adjusting for lactose intake, suggesting that lactose or other dairy components may act as prebiotics.
Conversely, higher cheese consumption correlated with a lower relative abundance of Bacteroides and Subdoligranulum. While Bacteroides have been implicated in colorectal cancer (CRC), lower levels of Subdoligranulum have been linked to metabolic disorders. Additionally, the study found that higher total dairy intake was negatively associated with Bacteroides, suggesting a complex relationship between dairy components and microbial composition. The varying impact of milk and cheese on gut microbiota composition may be due to differences in their nutrient content and fermentation process. Milk, which contains more lactose, may promote the growth of beneficial bacteria, while cheese, which undergoes fermentation, may have distinct effects on gut microbial communities.
The study did not find significant associations between yogurt intake and microbial composition, likely due to low yogurt consumption among participants. The findings suggest that different dairy products exert varying influences on gut microbiota, which may have implications for dietary recommendations and gut health interventions.
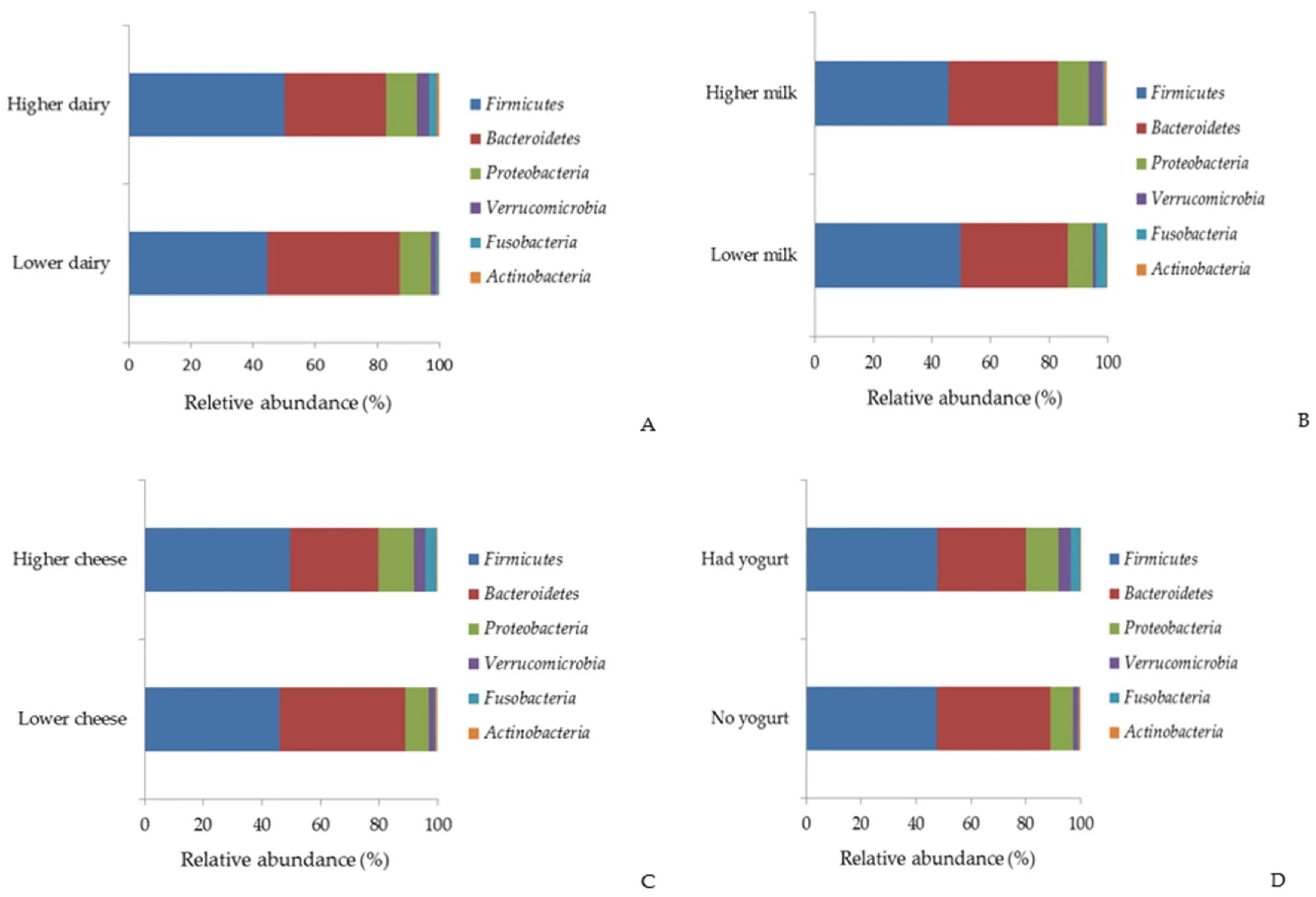 Relative abundance (%) of the major bacterial phyla by total dairy (A), milk (B), cheese (C), and yogurt (D).
Relative abundance (%) of the major bacterial phyla by total dairy (A), milk (B), cheese (C), and yogurt (D).
Conclusions
To summarize, dairy consumption significantly influences the composition and diversity of colonic mucosa-associated gut microbiota, with potential implications for individual and public health. A higher intake of total dairy and milk promotes beneficial bacteria such as Faecalibacterium and Akkermansia, whereas higher cheese consumption is linked to reductions in Bacteroides and Subdoligranulum. Notably, total dairy intake was inversely associated with Bacteroides, a genus linked to both colorectal cancer and inflammatory conditions. These findings underscore the broader impact of dairy consumption on gut health, which in turn affects metabolic, immune, and digestive functions.
On a community level, dietary guidelines emphasizing balanced dairy consumption could improve public health outcomes. However, the study had limitations, including a small sample size, a predominantly older male participant pool, and reliance on self-reported dietary intake, which may affect generalizability. Globally, understanding the role of dairy in gut health could inform nutrition policies, probiotic interventions, and personalized dietary recommendations. Further research using metagenomic and metabolomic approaches is needed to explore how specific dairy components influence microbial functions and their long-term effects on health.
Journal reference:
- Chen E, Ajami NJ, White DL, et al. Dairy Consumption and the Colonic Mucosa-Associated Gut Microbiota in Humans—A Preliminary Investigation. Nutrients. (2025), DOI: 10.3390/nu17030567, https://www.mdpi.com/2072-6643/17/3/567