Several modified vaccinia virus Ankara (MVA)-vectored vaccines are undergoing clinical trials against multiple pathogens, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In addition to intramuscular (IM) or subcutaneous administration, the IN administration of MVA has shown to induce protective mucosal and systemic immune responses in small animals, such as K18-human angiotensin-converting enzyme 2 (hACE2) mice.
It is noteworthy that MVA is a replication-deficient but immunogenic smallpox vaccine strain. In one of their previous studies, the authors demonstrated that IM injection of MVA expressing a modified SARS-CoV-2 spike (S) protein inactivated the furin cleavage site (FCS). This vaccine also induced neutralizing antibodies (nAbs) and the cluster of differentiation (CD8)+ interferon-gamma (IFN-γ)+ T cells to protect K18-hACE2 transgenic mice from SARS-CoV-2 infection. Also, sera from these mice passively transferred to unvaccinated mice protected against severe SARS-CoV-2-infection.
About the study
In the present study, researchers used a K18-hACE2 mouse model to compare the efficacies of IM and IN administration routes of a live, replication-deficient MVA–based SARS-CoV-2 S vaccine. Additionally, they determined whether vaccine-induced nAbs persisted for more than six months and that serum-induced to the Wuhan S protein neutralized pseudoviruses expressing S proteins of the SARS-CoV-2 Beta and Omicron variants.
The researchers modified a recombinant MVA expressing a SARS-CoV-2 S protein three times termed rMVA-Stri. To correlate vaccine dosage with the induced antibody levels, they varied the doses of rMVA-Stri from 2 × 103 to 2 × 107 plaque-forming units (PFU). The team administered these doses once, then twice again three weeks later. Moreover, they vaccinated control mice with the parental MVA.
The mice were challenged IN with a lethal dose of 105 median tissue culture infectious dose (TCID50) of Wuhan strain three weeks after the first vaccination and two weeks after the second. The researchers analyzed serum antibodies using an enzyme-linked immunosorbent assay (ELISA) three weeks after the prime and two weeks after the boost. They also monitored the SARS-CoV-2 S-binding and pseudovirus neutralizing titers over six to eight months following IM injection of 2 × 107 PFU of rMVA-Stri.
Study findings
The authors noted that IM vaccination-induced SARS-CoV-2 S-binding-nAbs and immunoglobulin G (IgGs) only in the lungs of infected mice. On the contrary, IN vaccination additionally induced IgA and higher levels of antigen-specific CD3+ CD8+ IFN-γ+ T cells. Notably, IgG was detected only in mice immunized IN; however, intranasal boosting increased IgA after both intranasal and intramuscular priming.
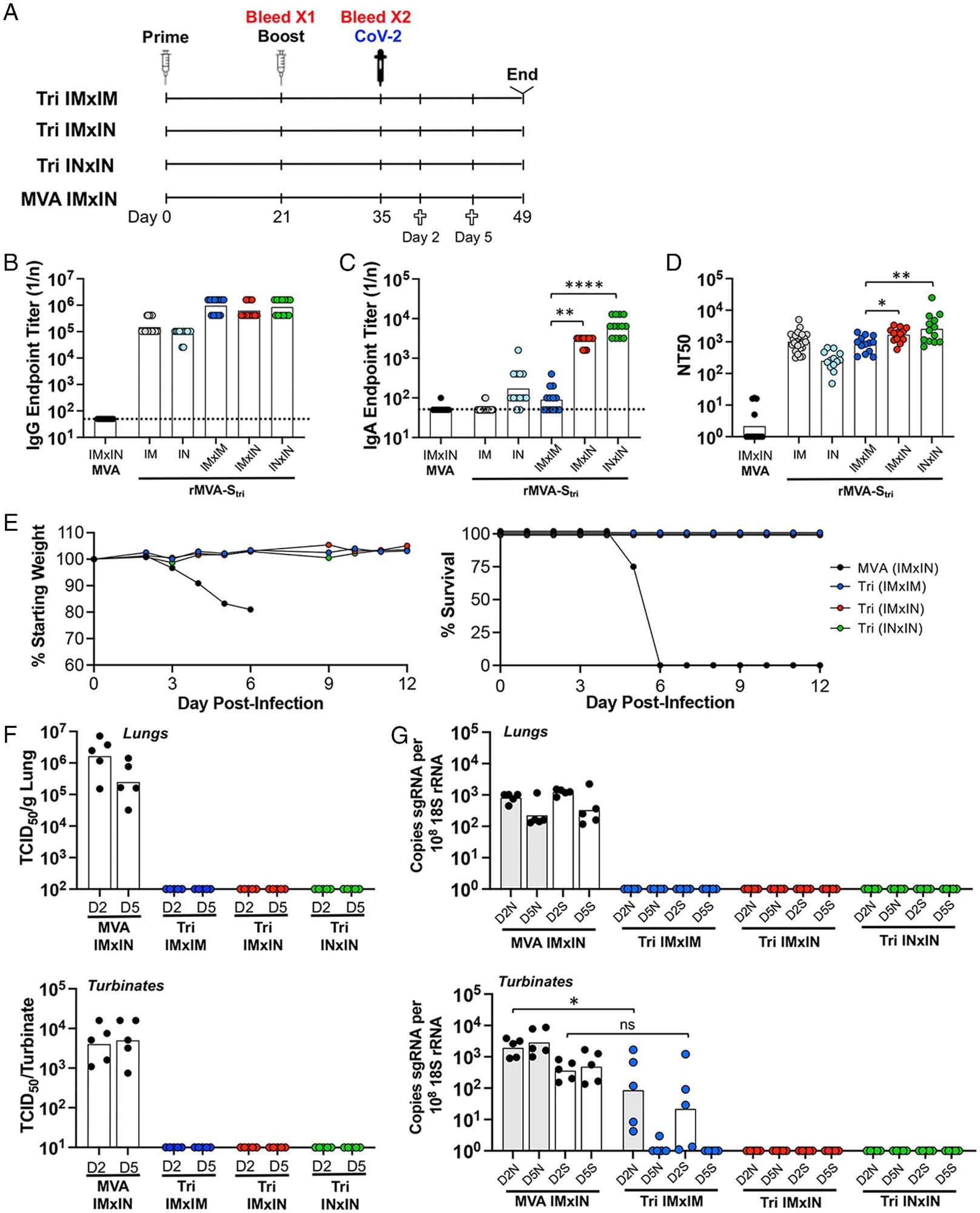 Comparison of immune responses and protection following IN and IM vaccinations. (A) K18-hACE2 mice were primed and boosted with rMVA-Stri by IN or IM route to form three groups of 13 mice: IMxIM, IMxIN, and INxIN. In addition, a control group received MVA IM followed by MVA IN. Mice from each group were bled at 3 wk after the prime and 2 wk after the boost. At the latter time, mice were challenged by IN inoculation of 105 TCID50 of SARS-CoV-2. On days 2 and 5 after challenge, five mice from each group were killed and three remained to follow weight and morbidity. (B and C) Serum IgG and IgA endpoint ELISA titers are shown for individual mice and the geometric mean. The dashed line represents the limit of detection. (D) Individual and geometric mean pseudovirus neutralizing titers are shown. (E) Three mice of each group were monitored for weight loss and survival. (F) At days 2 and 5 after challenge with SARS-CoV-2, homogenates were prepared from the lungs and nasal turbinates of five mice from each group and the TCID50 of SARS-CoV-2 determined. (G) RNA was extracted from the homogenates and copies of sgN (shaded) and sgS (unshaded) RNA were determined by ddPCR and normalized to 18S rRNA in the same sample. Significance: *P = 0.04, **P < 007, ****P < 0.0001; ns, not significant.
Comparison of immune responses and protection following IN and IM vaccinations. (A) K18-hACE2 mice were primed and boosted with rMVA-Stri by IN or IM route to form three groups of 13 mice: IMxIM, IMxIN, and INxIN. In addition, a control group received MVA IM followed by MVA IN. Mice from each group were bled at 3 wk after the prime and 2 wk after the boost. At the latter time, mice were challenged by IN inoculation of 105 TCID50 of SARS-CoV-2. On days 2 and 5 after challenge, five mice from each group were killed and three remained to follow weight and morbidity. (B and C) Serum IgG and IgA endpoint ELISA titers are shown for individual mice and the geometric mean. The dashed line represents the limit of detection. (D) Individual and geometric mean pseudovirus neutralizing titers are shown. (E) Three mice of each group were monitored for weight loss and survival. (F) At days 2 and 5 after challenge with SARS-CoV-2, homogenates were prepared from the lungs and nasal turbinates of five mice from each group and the TCID50 of SARS-CoV-2 determined. (G) RNA was extracted from the homogenates and copies of sgN (shaded) and sgS (unshaded) RNA were determined by ddPCR and normalized to 18S rRNA in the same sample. Significance: *P = 0.04, **P < 007, ****P < 0.0001; ns, not significant.
Moreover, IN vaccination rapidly cleared SARS-CoV-2 infection as early as two days after the live virus challenge. Accordingly, the authors could not detect the infectious virus or viral messenger ribonucleic acid (RNAs) in the nasal turbinates or lungs of the infected mice, even two days after the virus challenge. Conversely, IM vaccination cleared SARS-CoV-2 from the respiratory tract several days after the challenge.
ELISA results indicated that around 50% of the mice had higher S-binding antibody titers than the control mice after priming with the 2 × 103 PFU of rMVA-Stri, whereas nearly all mice had elevated titers after the boost. The researchers detected binding antibodies at a higher dose in all mice after the priming. Moreover, the endpoint titers were similar after the boost except for the 2 × 104 PFU dose, and the boosted values were similar following doses of 2 × 104 PFU or higher.
The test animals that received a single lower dose died by day six or seven, whereas those who received a booster dose survived. Overall, survival correlated with a 50% neutralization titer (NT50) of ∼100 and a single dose of 2 × 106 PFU. The booster dose of 2 × 105 PFU or higher raised these values by 10- to 100-fold.
Furthermore, the decrease in binding antibody titer was 19% and 75% at four weeks and over six months, respectively. Likewise, the nAb titers declined 22% and 81% at four weeks and over six months, respectively, but remained adequate to correlate with protection. Mann-Whitney test showed a significantly higher NT50 after the second vaccination received three weeks later than the first one, supporting the advantage of delaying a booster dose when appropriate.
Conclusions
The study focused on a comparison of IN and IM routes of administration for MVA-based COVID-19 vaccines. Two IN vaccinations cleared SARS-CoV-2 single guide RNAs (sgRNAs) in the nasal turbinates and lungs of mice within two days, indicating how it efficiently accelerated viral clearance likely by recruiting local IgA antibodies. Notably, the polymeric structure of secretory IgA antibodies helps potently neutralize SARS-CoV-2.
Recently, some countries, including the United States, Canada, and the European Union, have approved MVA as a smallpox vaccine. Although rMVA-vectored vaccines have not received approvals for COVID-19 yet, the present study supports testing MVA-vectored SARS-CoV-2 vaccines, including their IN delivery routes and subsequent infection and transmission rates.