The choice of CCP for therapy is guided by the high titers of immunoglobulin G (IgG) against the spike (S) protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and neutralization titers. The neutralizing antibodies (nAbs) in CCPs could inactivate SARS-CoV-2. Evidence suggests that CCP administration reduces inflammation and helps alleviate SARS-CoV-2-induced acute respiratory disorder syndrome (ARDS). Nevertheless, several large randomized controlled trials (RCTs) have found limited-to-no evidence in lowering the risk of intubation and death.
However, reports suggest that CCP should be administered early in the disease course and must have the highest neutralization titers. CCPs contain an ensemble of polyclonal antibodies with varying epitope specificity and neutralizing and non-neutralizing activities. Additionally, antibodies could use their Fc domain to mediate effector functions by engaging with Fc receptors (FcRs) on innate immune cells. FcR interaction could result in viral clearance through antibody-dependent phagocytosis (ADP) or antibody-dependent cellular cytotoxicity (ADCC).
The study and results
In the current study, researchers screened CCPs using lethally challenged SARS-CoV-2-infected K18-hACE2 mice. Bioluminescence imaging (BLI) was used to track SARS-CoV-2 infection.
CCPs with low nAb titers collected during the first COVID-19 wave were selected and classified based on in vitro ADCC activity as CCP-1 to CCP-6. K18-hACE2 mice were challenged with a lethal dose of SARS-CoV-2 WA1 strain under prophylactic and therapeutic regimens. CCP was administered 24 hours before challenge and two days post-infection (dpi) in mice under prophylaxis and therapy, respectively.
Analyses showed that the administration of CCP-6 controlled viral replication in the lungs, brain, and nose, preventing body weight loss and mortality. Mice under prophylaxis with CCP-3, 4, or 5 initially lost weight but began recovering by 7 dpi and eventually had viral replication under control. Contrastingly, viral replication was uncontrolled in mice receiving CCP-1 or 2, and the animals succumbed by 6 dpi. Three out of four CCPs (with ADCC activity) rescued mice in the therapeutic regimen from SARS-CoV-2-induced death.
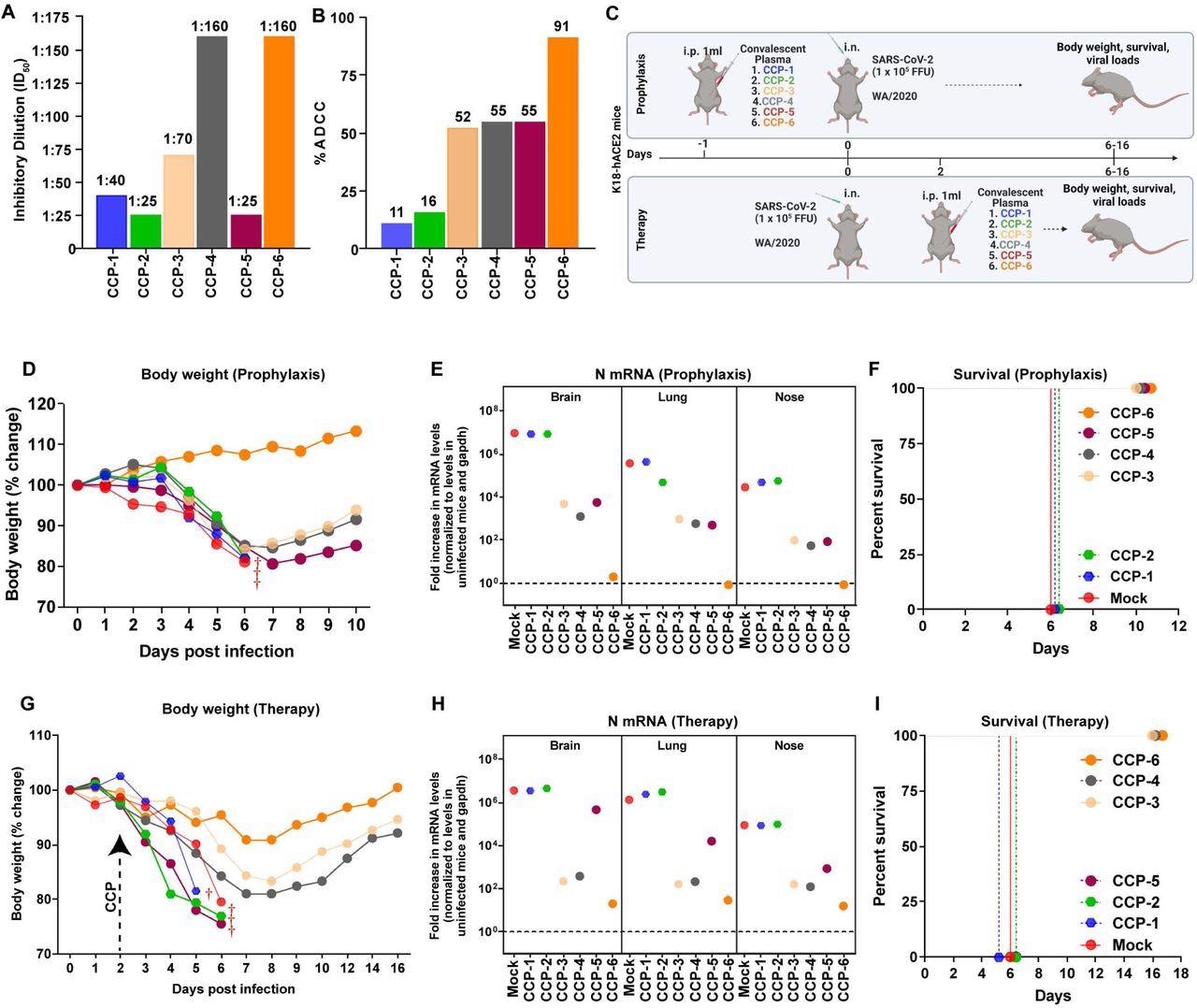
In vivo screening of COVID-19 Convalescent Plasma (CCPs) (A) A graph depicting WA1-neutralizing activity of indicated CCPs expressed as inhibitory dilution of plasma (ID50) that reduces FFUs by 50% using Vero E6 cells as targets. (B) %ADCC in the presence of CCP using a 1:1 ratio of parental CEM.NKr cells and CEM.NKr.Spike cells as target cells while PBMCs from uninfected donors were used as effector cells. (C) A scheme showing experimental design for screening in vivo efficacy of indicated CCPs delivered 1ml per 20-25 g body weight of mouse intraperitoneally (i.p.) under prophylaxis (-1dpi) and therapeutically (+2 dpi) in K18-hACE2 mice intranasally (i.n.) challenged with 1 x 105 FFU WA1 SARS-CoV-2-nLuc. PBS-treated mice were used as control (Mock). (D, G) Temporal changes in mouse body weight with initial body weight set to 100% during CCP prophylaxis (-1dpi) and therapy (+2 dpi) for experiment as in C. (E, H) Fold change in SARS-CoV-2 nucleocapsid (N gene) expression in brain, lung and nose tissues during CCP prophylaxis and therapy for experiment shown in C. The data were normalized to Gapdh mRNA expression in the same sample and that in non-infected mice after necropsy. (F, I) Kaplan-Meier survival curves for evaluating in vivo efficacy of CCPs against SARS-CoV-2- nLuc for an experiment as in C.
The authors continued further investigations with CCP-6, whose protective profile was contingent on neutralization and Fc-effector functions. CCP-2 was also used, which had lower ADCC and neutralizing activities than CCP-6. Mice were prophylactically administered with CCPs a day before infection with nanoluciferase (nLuc) SARS-CoV-2.
Temporal BLI imaging and nLuc signal quantification revealed that prophylaxis with CCP-2 (or human IgG1 in control mice) failed to prevent SARS-CoV-2-nLuc infection and subsequent spread/replication. Contrastingly, prophylaxis with CCP-6 completely prevented viral infection, as inferred by the absence of nLuc signals.
Under therapeutic mode, mice that received CCP-2 or IgG1 two days after the SARS-CoV-2-nLuc challenge failed to control viral replication in the lungs, and the infection spread to the brain. In contrast, CCP-6 recipients cleared the lung infection by 8 dpi. Interestingly, although neuro-invasion was evident at 6 dpi, CCP-6 therapy controlled and cleared viral particles in the brain by 10 dpi.
Next, the mice were depleted of macrophages or neutrophils using depleting antibodies, and flow cytometry confirmed the depletion of about 98% neutrophils in blood and 75% lung-resident macrophages. When CCP-6 was administered under prophylaxis in these immuno-depleted mice, their susceptibility to SARS-CoV-2 infection was unaltered. BLI investigations revealed weak and ephemeral viral replication in lungs at 4 and 6 dpi, which was cleared by 10 dpi. A temporary body weight loss was also noted in these mice relative to mice without immune depletion. The inflammatory cytokine levels were also higher than in non-depleted mice.
The same experiments involving immune depletion were repeated under the therapeutic mode, and the results were drastic. The authors noted that CCP-6-mediated virologic control was significantly compromised, and the immune-depleted mice lost 20% – 30% body weight before succumbing to infection. Half the mice in the macrophage-depleted cohort and all animals in neutrophil-depleted cohorts showed a one-day delay in death.
Neuro-invasion was also evident by 6 dpi in 50% and 75% of mice in macrophage- and neutrophil-depleted cohorts. This indicated that innate immune system-mediated Fc-effector functions contributed significantly to the protection conferred by CCP-6 during therapy. In subsequent experiments, CCP-6 was depleted of IgG or IgM + IgA antibodies. Although both depleted fractions retained neutralizing activity, the IgM + IgA-depleted fraction (which had IgG) exhibited a 2.3-fold more significant neutralization than the IgG-depleted fraction.
When the in vivo efficacy was assessed during prophylactic administration of antibody-depleted CCP-6, the researchers noted a complete loss of protection with IgG-depleted fraction. In comparison, IgM + IgA-depleted CCP-6 fraction showed virologic control similar to non-depleted CCP-6.
Intriguingly, when neutrophils were depleted, and the IgM + IgA-depleted fraction was used for prophylaxis, viral replication was not controlled, and SARS-CoV-2 disseminated into the brain by 8 dpi, and all mice succumbed to infection. During therapy, the efficacy of IgG or IgM + IgA depleted CCP-6 was severely compromised. Only one mouse in each IgG- and IgM + IgA-depleted cohort survived.
Lastly, live virus neutralization experiments revealed that all CCPs, except CCP-1 and CCP-5, had similar neutralizing activity against SARS-CoV-2 WA1 and Delta variants. Still, the neutralizing activity against the Beta variant was significantly diminished for all CCPs. They tested the in vivo efficacy of these CCPs to protect against Beta or Delta variant infection under prophylaxis and therapy.
Prophylaxis with CCP-1 or 2 failed to protect against Delta infection, and all mice succumbed by 6 dpi. CCP-4 and CCP-6 protected mice against Delta infection and prevented death. However, CCP-3 and CCP-5 administration only delayed mortality by two and three days. None of the CCPs under prophylaxis protected against Beta infection-induced death. Also, none of the CCPs under therapeutic mode prevented mortality upon Beta or Delta variant infection.
Conclusions
In summary, the study demonstrated that CCPs with low neutralizing potential but robust Fc effector functions could protect against homologous SARS-CoV-2 infection under prophylaxis and therapy. IgA, IgG, and IgM were required for optimal virologic control. Despite the decreased neutralizing activity, CCPs provided some degree of cross-protection against SARS-CoV-2 Beta and Delta variants by delaying mortality likely through Fc-effector functions. These findings support that Fc-effector functions be considered in addition to neutralizing potency in selecting CCPs for therapy.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
The Fc-effector function of COVID-19 convalescent plasma contributes to SARS-CoV-2 treatment efficacy in mice , Irfan Ullah, Guillaume Beaudoin-Bussières, Kelly Symmes, Marc Cloutier, Eric Ducas, Alexandra Tauzin, Annemarie Laumaea, Philippe Bégin, Walther Mothes, Priti Kumar, Renée Bazin, Andrés Finzi, Pradeep D. Uchil, bioRxiv 2022, https://doi.org/10.1101/2022.06.10.495677, https://www.biorxiv.org/content/10.1101/2022.06.10.495677v1
- Peer reviewed and published scientific report.
Ullah, Irfan, Guillaume Beaudoin-Bussières, Kelly Symmes, Marc Cloutier, Eric Ducas, Alexandra Tauzin, Annemarie Laumaea, et al. 2023. “The Fc-Effector Function of COVID-19 Convalescent Plasma Contributes to SARS-CoV-2 Treatment Efficacy in Mice.” Cell Reports Medicine 4 (1). https://doi.org/10.1016/j.xcrm.2022.100893. https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(22)00472-4.