The significantly transmissible SARS-CoV-2 Omicron variant, initially discovered in South Africa in November 2021, had become the dominant viral variant in the US at the end of December 2021. The surges in SARS-CoV-2 cases caused by Omicron and its emerging BA.1 and BA.2 lineages were the greatest ever encountered in the US, with a peak of above 400,000 patients per day.
Mounting evidence on coronavirus disease 2019 (COVID-19) vaccine protection against Omicron infection is equivocal. Furthermore, uncertainties exist about the efficacy of SARS-CoV-2 vaccine booster doses and whether COVID-19 booster doses help particular demographic and at-risk groups the most. Moreover, determining the effectiveness of COVID-19 vaccinations against serious illnesses caused by the Omicron mutant and its lineages has been difficult, which may explain the wide range of recent results.
About the study
In the current study, the researchers compared the efficacy of a primary SARS-CoV-2 vaccine regimen and a booster dose versus a primary vaccination regimen alone in preventing the COVID-19 hospitalization linked with the SARS-CoV-2 Omicron variant. The team used a test-negative strategy to examine vaccine effectiveness (VE) in the present multicenter observational case-control research in 21 hospitals across the US.
The authors aimed to determine the efficacy of COVID-19 vaccine booster doses and the interaction between immune escape and declining immunity in the Omicron surge. Additionally, they assessed serum antibody titers in healthy adult participants to evaluate anti-SARS-CoV-2 reactions pre- and post-receipt of booster doses as a complementing laboratory investigation to these clinical VE studies.
The study consisted of 3,181 adults admitted to the hospital with an acute respiratory disease between 26 December 2021 and 30 April 2022, a period of SARS-CoV-2 Omicron BA.1 and BA.2 variant predominance. There were 1,572, i.e., 49%, case-patients with laboratory-verified SARS-CoV-2 infection and 1,609, i.e., 51%, control individuals who tested negative for COVID-19.
The key outcome measure, VE against SARS-CoV-2-associated hospitalization, was estimated for a primary vaccination series followed by a booster dose and a primary vaccination regimen only by comparing the chances of being vaccinated with each series relative to being non-vaccinated between cases versus controls. VE analyses were segregated based on immune status, i.e., immunocompetent or immunocompromised since the recommended vaccination schedules for these populations varied. The core analysis assessed all COVID-19 vaccine kinds merged, whereas supplementary investigations evaluated individual vaccine products.
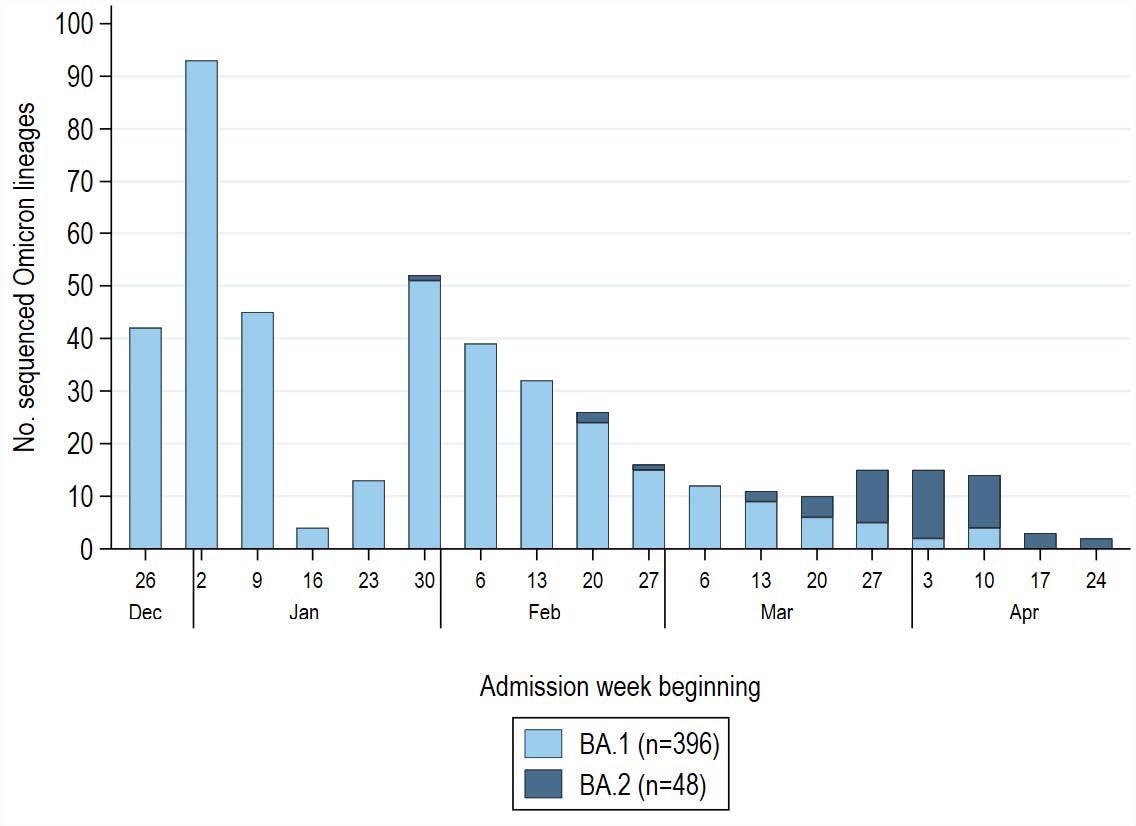
SARS-CoV-2 Omicron lineage by admission week among 1,572 COVID-19 case-patients enrolled during December 26, 2021–April 30, 2022 (with a pause in enrollment January 25–31, 2022). Sequenced Omicron variants were grouped into BA.1 (B.1.1.529, BA.1, BA.1.1, BA.1.15, BA.1.17) and BA.2 (BA.2, BA.2.1, BA.2.3) lineages. Non-Omicron case-patients (Delta variant, B.1.1.519) confirmed through sequencing were excluded from the analysis (n=64) and not displayed in this figure. Of 444 patients with a sequence-confirmed Omicron variant infection, 396 (89%) had BA.1 and 48 (11%) had BA.2. Low sequencing totals in late January reflect a pause in IVY network enrollment during January 25–31, 2022 during a protocol update.
Results
The study results demonstrated that the median age of the study volunteers was 64, 21% were immunocompromised, and 48% were women. In addition, 798, i.e., 25%, were immunized with a primary COVID-19 vaccination series plus booster dose; 1,326, i.e., 42%, were vaccinated with a primary vaccination regimen only; and 1,057, i.e., 33%, were non-vaccinated.
VE against Omicron-linked COVID-19 hospitalization in immunocompetent individuals was 77% for a primary vaccine regimen combined with one booster dose of any vaccine product and 44% for a primary series only. The VE for a primary series of any vaccine formulation versus Omicron-related COVID-19 hospitalization was 60% in immunocompromised individuals. Inadequate sample size prevented estimation of boosted schedules effectiveness among immunocompromised participants.
Boosted vaccination schedules of the two messenger ribonucleic acid (mRNA) vaccines administered in the US had higher VE than a primary regimen alone. The VE of the SARS-CoV-2 BNT162b2 vaccine primary series plus booster dose was 80%, whereas VE for the primary series alone was 46%. Further, while the COVID-19 mRNA-1273 vaccine primary series plus booster VE was 77%, the primary series alone VE was 47%.
Besides, SARS-CoV-2 mRNA vaccines (mRNA-1273 and BNT162b2) gave better protection than the Ad26.COV2 vaccine in both boosted vaccine regimes and primary series alone. Further, serology data showed significantly greater anti-SARS-CoV-2 antibody levels after booster vaccination doses, confirming the clinical VE results.
Overall, the findings suggested that receiving a COVID-19 primary vaccine regimen plus a booster dose gave better protection from COVID-19-related hospitalization than receiving only a primary vaccine course. This elevated immunity by booster vaccine dose was observed in all age categories and for all three vaccine formulations employed in the US.
Conclusions
The study findings depicted that a booster dose of the SARS-CoV-2 vaccine conferred extra benefits above a primary vaccine series alone in reducing Omicron-linked hospitalization in US populations. Additionally, SARS-CoV-2 mRNA vaccines, both as booster regimens and a primary series, outperformed the Ad26.COV2 vaccine. Serology results reveal significant hikes in anti-SARS-CoV-2 antibody levels after booster vaccine doses, especially with mRNA vaccines, corroborating the findings.
Collectively, the present findings back up the advice that all eligible people in the US aged 18 years or older receive an mRNA vaccine booster dose to protect them from the Omicron variant's severe illness.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Vaccine Effectiveness of Primary Series and Booster Doses against Omicron Variant COVID-19-Associated Hospitalization in the United States; Katherine Adams, Jillian P. Rhoads, Diya Surie, Manjusha Gaglani, Adit A. Ginde, Tresa McNeal, Shekhar Ghamande, David Huynh, H. Keipp Talbot, Jonathan D. Casey, Nicholas M. Mohr, Anne Zepeski, Nathan I. Shapiro, Kevin W. Gibbs, D. Clark Files, Madeline Hicks, David N. Hager, Harith Ali, Matthew E. Prekker, Anne E. Frosch, Matthew C. Exline, Michelle N. Gong, Amira Mohamed, Nicholas J. Johnson, Vasisht Srinivasan, Jay S. Steingrub, Ithan D. Peltan, Samuel M. Brown, Emily T. Martin, Arnold S. Monto, Adam S. Lauring, Akram Khan, Catherine L. Hough, Laurence W. Busse, Caitlin C. ten Lohuis, Abhijit Duggal, Jennifer G. Wilson, Alexandra June Gordon, Nida Qadir, Steven Y. Chang, Christopher Mallow, Carolina Rivas, Hilary M. Babcock, Jennie H. Kwon, James D. Chappell, Natasha Halasa, Carlos G. Grijalva, Todd W. Rice, William B. Stubblefield, Adrienne Baughman, Christopher J. Lindsell, Kimberly W. Hart, Sandra N. Lester, Natalie J. Thornburg, SoHee Park, Meredith L. McMorrow, Manish M. Patel, Mark W. Tenforde, Wesley H. Self. medRxiv preprint 2022, DOI: https://doi.org/10.1101/2022.06.09.22276228, https://www.medrxiv.org/content/10.1101/2022.06.09.22276228v1
- Peer reviewed and published scientific report.
Adams, Katherine, Jillian P Rhoads, Diya Surie, Manjusha Gaglani, Adit A Ginde, Tresa McNeal, H Keipp Talbot, et al. 2022. “Vaccine Effectiveness of Primary Series and Booster Doses against Covid-19 Associated Hospital Admissions in the United States: Living Test Negative Design Study.” BMJ, October, e072065. https://doi.org/10.1136/bmj-2022-072065. https://www.bmj.com/content/379/bmj-2022-072065.