The development of the microbiome in humans is closely related to, and forms a crucial part of, the maturation of the human immune system. Vaginal childbirth is one of the first steps of this process, as it promotes the transmission of beneficial bacterial species from the mother to the baby during labor that continues during parturition and breastfeeding.

Study: Mother–Infant Transmission of Human Microbiota. Image Credit: Alena Ozerova / Shutterstock.com
Introduction
The human organism is typically sterile in utero and receives a wide range of microorganisms from its local environment and mother that will eventually colonize various body sites within the infant. Over time, the pioneer taxa give rise to a ‘climax community,’ which consists of microbial species that are adapted to the host, able to resist invading organisms and prevent pathogenic overgrowth, all the while contributing to the overall health of the host.
While the bacterial transmission is an integral part of bacterial persistence, as it ensures that the microorganisms continue to exist by colonizing new hosts, the usual mechanisms like spore formation, tolerance of air exposure, and thickened cell walls do not function in gut bacteria.
Important maternal species
Several microbial species have been reported to be transmitted from the mother to the infant during vaginal delivery. These same species have been detected on other sites of the mother’s body including breast milk and the skin, as well as in the infant's feces during the perinatal period.
Some of these initial species include Bacteroidetes and Actinobacteria. Notably, several Bifidobacterium and Bacteroides species break down human milk oligosaccharides (HMO) to allow the infant to digest them.
Although Bacteroides dominate the gut microbiome (GM) in adults, these species differ from those found in infants. These species are able to adapt better to immunological and gut environmental changes as the infant changes into an adult.
Furthermore, the Bacteroides found within the infant gut exhibit unique characteristics such as a number of enzymes that break down complex carbohydrates, gene switches that can promote operon transcription to regulate bacterial cell surface-associated products, as well as certain mutations that make the cell envelope favorable for living and proliferating in the gut.
Phylum Firmicutes is also found in abundance in the adult gut. However, of the 11 species transmitted from the mother to the infant, only two persist after the first month of life. Spore formation is minimal, perhaps because an essential component for such species to flourish is missing from the infant's gut.
In fact, breastmilk may favor bacteria that metabolize HMOs. However, once breastfeeding stops, spore-formers begin to replace these species, which may lead to the spread of other sporulating bacteria from family members or other environmental factors. Thus, these bacteria are not particularly dependent upon maternal transmission.
![Phylogenetic distribution of maternally transmitted gut bacteria in the neonatal period. A representative phylogeny of human gut bacteria found in infants and adults based on cultured isolates [28]. The main bacterial phyla in the human gut are represented by blue shades. The red-filled dots represent species that are reported as maternally transmitted in the neonate period (< 1 month) and persist beyond, red unfilled dots represent species that are maternally transmitted but do not persist beyond this period. The absence of a red dot indicates that the persistence level is unknown. Determination of persistence is based on the absence of species based on sequencing or culturing technologies and therefore, we cannot exclude the possibility they may still be present at extremely low abundance levels. Colored bars represent the maternal reservoir. A strong phylogenetic signal is present as the maternal transmission is dominated by Bacteroidetes and Actinobacteria bacteria derived from the maternal gut.](https://www.news-medical.net/images/news/ImageForNews_718596_16570732759971992.jpg)
Phylogenetic distribution of maternally transmitted gut bacteria in the neonatal period. A representative phylogeny of human gut bacteria found in infants and adults based on cultured isolates [28]. The main bacterial phyla in the human gut are represented by blue shades. The red-filled dots represent species that are reported as maternally transmitted in the neonate period (< 1 month) and persist beyond, red unfilled dots represent species that are maternally transmitted but do not persist beyond this period. The absence of a red dot indicates that the persistence level is unknown. Determination of persistence is based on the absence of species based on sequencing or culturing technologies and therefore, we cannot exclude the possibility they may still be present at extremely low abundance levels. Colored bars represent the maternal reservoir. A strong phylogenetic signal is present as the maternal transmission is dominated by Bacteroidetes and Actinobacteria bacteria derived from the maternal gut.
Routes of microbial transmission
The primary routes of microbial transmission are from the mother’s gut, which contributes almost 30 species, and the skin, and the tongue, each of which contributes seven and five species respectively. The vagina and breastmilk also contribute less than five microbial species each to the infant.
Most of the species from the skin and tongue die out early, except for Veillonella parvula. However, Veillonella are very common in the infant's gut and feed on lactate supplied by Streptococcus or Lactobacillus to generate short-chain fatty acids (SCFAs) such as propionate and acetate. This suggests an interdependent relationship, such that “their abundance in the early-life gut microbiota may be due to cross-feeding with lactic acid-producing bacteria that consume HMOs.”
Lactobacilli have also been reported by earlier studies to be primarily supplied to the infant from the mother’s vagina during the delivery. Nevertheless, only three species were found in the current study to be vaginally derived, all of which did not survive past the neonatal period.
Three Bifidobacterium species were traced to breast milk and the maternal gut. It is not clear how gut bacteria reach breast milk; however, researchers have proposed the existence of an enteromammary route that may transport gut-derived bacteria from the mother’s gut to the breast milk through immune cells. Interestingly, some of the Bifidobacterium species found in breast milk, such as B. infantis and B. breve, are never found in the adult gut.
The maternal gut remains the source of all persistent species to the infant, thus indicating that these microorganisms are important for the development of the infant gut. The time of transmission may be at birth; however, it is more likely that the transmission of these microorganisms occurs after delivery, likely through the feco-oral route. Over time, the infant gut microbiome profile will more closely resemble that of the adult gut.
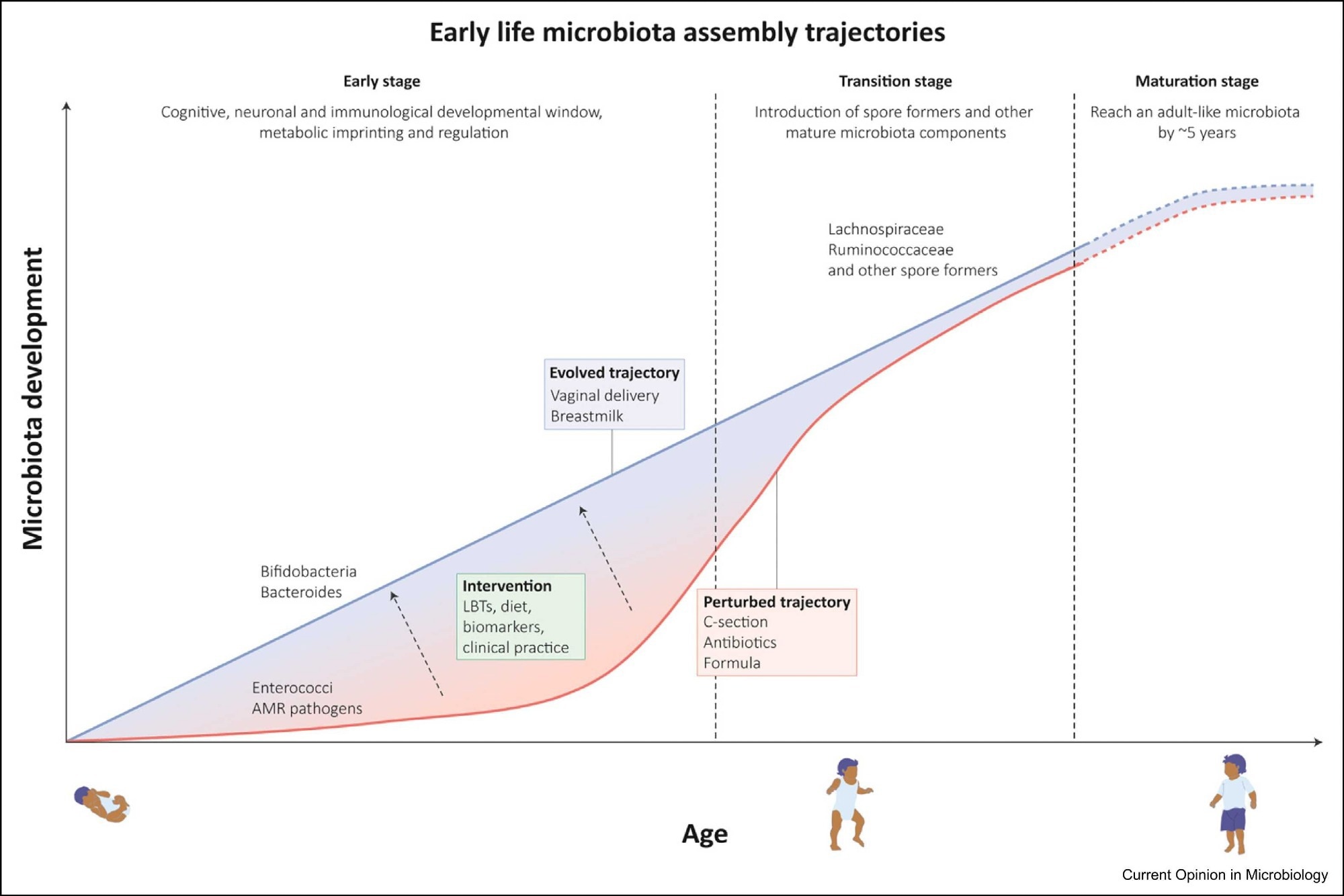 Early-life microbiota assembly trajectories: Gut microbiota acquisition commences at birth and rapidly increases in composition and functional capacity. This process is marked by different stages, (1) an early stage that lasts until 6–12 months when weaning occurs (exact times remain unknown) and which acts as a developmental window, important to immunological and physiological development (2) a transition stage associated with cessation of breastfeeding and introduction of solid foods leading to colonization by diverse spore-forming species and a reduction in Bifidobacterium species (3) maturation stage which resembles an adult-like composition abundant in Bacteroidetes and Firmicutes species. Microbiota perturbations impact this assembly trajectory by preventing transmission and colonization of beneficial species and instead lead to colonization of opportunistic pathogens. Therapeutic interventions through Live Bacterial Therapeutics (LBT) or by modulating diet hold promise to restore and select for age-appropriate beneficial species.
Early-life microbiota assembly trajectories: Gut microbiota acquisition commences at birth and rapidly increases in composition and functional capacity. This process is marked by different stages, (1) an early stage that lasts until 6–12 months when weaning occurs (exact times remain unknown) and which acts as a developmental window, important to immunological and physiological development (2) a transition stage associated with cessation of breastfeeding and introduction of solid foods leading to colonization by diverse spore-forming species and a reduction in Bifidobacterium species (3) maturation stage which resembles an adult-like composition abundant in Bacteroidetes and Firmicutes species. Microbiota perturbations impact this assembly trajectory by preventing transmission and colonization of beneficial species and instead lead to colonization of opportunistic pathogens. Therapeutic interventions through Live Bacterial Therapeutics (LBT) or by modulating diet hold promise to restore and select for age-appropriate beneficial species.
Immunological impact of infant colonization
It is well-recognized that Cesarean section and the use of antibiotics are sometimes necessary. Unfortunately, these events are also associated with severe disruption of natural processes that lead to the establishment of a healthy infant gut microbiome.
In the absence of normal maternal-infant transmission, there is an increased risk that opportunistic pathogens including Proteobacteria such as Enterococcus and Klebsiella from the hospital or other environmental sources following Cesarean sections can harm the infant.
Comparatively, the use of antibiotics reduces the transmission rate of maternal microbes to the infant, thus impacting the development of the infant gut microbiome. At any given age, infants who have received antibiotics are less likely to have the optimal diversity of gut microbes as compared to those who have not.
Interestingly, asthma is more prevalent following both Cesarean section and antibiotics in infancy. This suggests that early life interventions that perturb microbiome development can also have an impact on the development of the immune system.
During infancy, innate immunity, including dendritic cells and natural killer cells, mature. This is related to microbial colonization of the gut during the first three months of life. Exposure to these antigens induces immune tolerance, provided that this exposure occurs within the first few weeks of life.
If exposure to immunomodulatory bacteria in the gut like Bifidobacterium and Bacteroides occurs after this period, a pro-inflammatory response is initiated, rather than tolerance. “This suggests a developmental window is present in humans, during which key immune system-microbial interactions lead to normal immune development.”
B. infantis promotes normal immunologic development by subsequently promoting the expansion of T regulatory cells (Treg); however, this species decreases the frequency of pro-inflammatory type 2 Th2 cells. With breastfeeding, B. infantis and other Bifidobacterium species generate metabolites like aromatic lactic acids. These activate the aryl hydrocarbon receptor, thus causing immunomodulation.
In sterile mouse experiments, Bacteroides fragilis from the mother’s gut produces cell surface capsular polysaccharides. This leads to the development of lymphoid and CD4 T-cells, which help cytotoxic CD8 T-cells eliminate pathogens.
Implications
Maternal transmission of gut bacteria provides a microbial ‘starter kit’ for infants which promotes healthy growth and disease resistance.”
The current study suggests that both Bifidobacterium and Bacteroides species likely play an important role in the development of the immune system. Furthermore, these species appear to modulate this system through multiple chemicals produced during their metabolism that subsequently affect the growth, maturation, and activity of the brain and immune system.
Further detailed studies on in vitro cultures and metabolomics will be necessary to better understand the role of these bacteria in the human organism. These studies should subsequently be repeated and extended in different populations, many of which lack basic GM profiling.
In the future, such studies will also provide insight into how the gut microbiota can be restored to its normal rate of development once it is disturbed.
Since the infant GM is simpler and less diverse than the adult GM, the development of precise therapeutic mixtures of microbes should be easier. This could be in the form of prebiotics that enhance the growth of beneficial species, or through the introduction of live bacterial therapeutic (LBT), which is a mix of suitable bacterial species.
The determination of appropriate therapeutic measures for this purpose requires knowledge of which microorganisms are locally transmitted species, as these are more likely to support the development of the infant GM as compared to non-native species that are not appropriate to the local diet, customs, and environment.
These optimized mixtures may help treat malnutrition or necrotizing enterocolitis, as well as potentially prevent asthma or allergies due to abnormal immune development. By nurturing maternally-derived beneficial species, such approaches may help repair the harm done by the absence of the “microbial starter kit” in neonatal life.