A new set of strategies is being investigated to combat the coronavirus disease 2019 (COVID-19), as well as other future viral pandemics. This includes the use of novel antigens, adjuvants, and delivery systems.
Study: Circular RNA Vaccine, A Novel mRNA Vaccine Design Strategy For SARS-Cov-2 And Variants. Image Credit: Christoph Burgstedt / Shutterstock.com
Introduction
The COVID-19 pandemic was caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which enters the host cell through the use of its viral spike antigen. To this end, the receptor binding domain (RBD) within the SARS-CoV-2 spike protein binds to the host cell angiotensin-converting enzyme 2 (ACE2) receptor.
The devastating nature of the COVID-19 pandemic fueled global research efforts, which culminated in the production and emergency authorization of several COVID-19 vaccines within a year of the onset of the outbreak.
Some of the most notable COVID-19 vaccines included those that were built on the messenger ribonucleic acid (mRNA) platform. These vaccines were found to induce efficient neutralizing antibodies (nAbs) and type 1 T helper cell (Th1) responses, along with antiviral effector cellular responses that involved interferon γ (IFN-γ+) CD8+ T-cells.
However, such vaccine platforms have some inherent defects, as they are extremely heat-labile and, as a result, require stringent and expensive manufacturing processes. This characteristic of mRNA vaccines has also presented logistical challenges for storage and transport, thus rendering them unsuitable for low-resource settings.
The mRNA vaccines present many technical challenges, particularly because of their vulnerability to heat-induced denaturation and enzymatic degradation by exonuclease RNase R. Thus, these vaccines must be manufactured in RNase-free environments and under very sterile conditions.
Moreover, linear mRNA requires the addition of a 5` cap and 3` poly-adenine (polyA) tail, with modified nucleotides such as 1-methylpseudouridine (1mΨ) to ensure that exonuclease digestion does not occur. The use of lipid nanoparticles (LNPs) to encapsulate the mRNA offered additional protection.
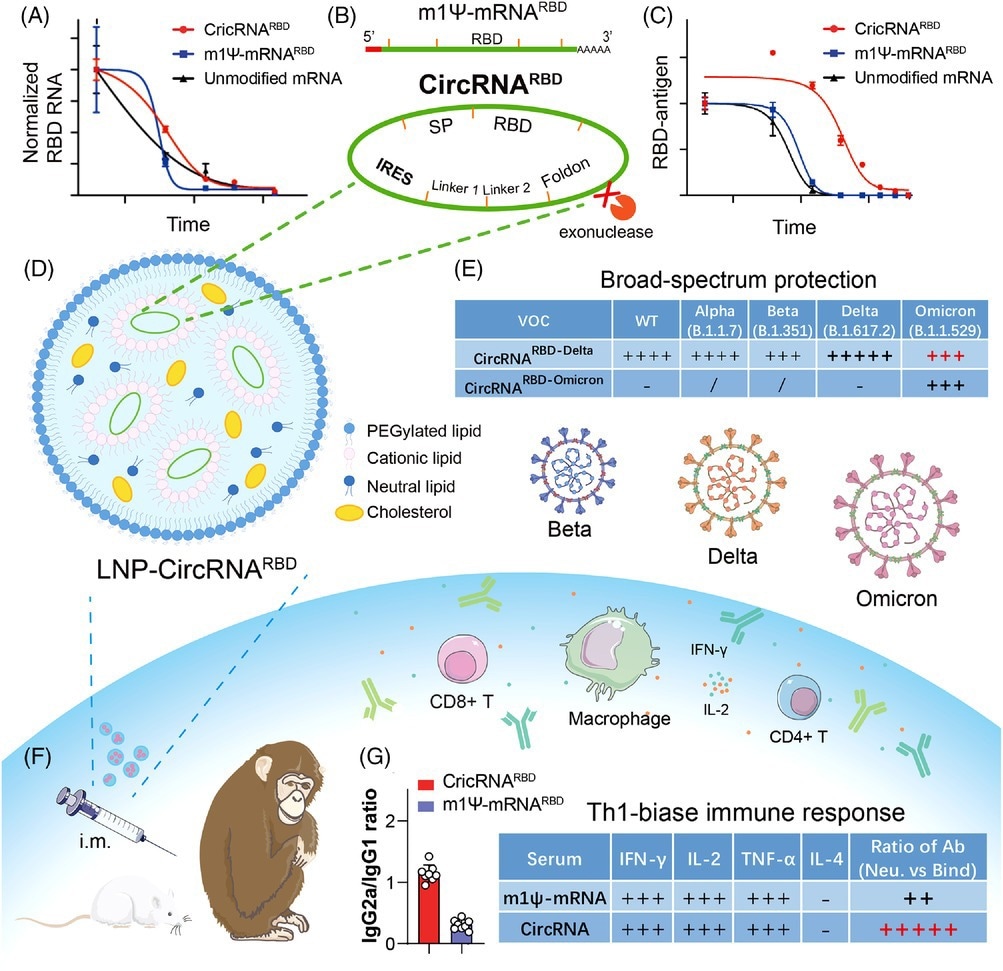
The design strategy and major advantages of circRNA vaccine against SARS-CoV-2 and variants. (A and C) Compared with m1Ψ-mRNARBD, circRNARBD has longer half-life and more durable antigen expression. (B) IRES element is introduced to initiate the translation of SARS-CoV-2 RBD antigen. (D, F, and G) LNP-encapsulated circRNA vaccines injected intramuscularly into mice and rhesus macaques induce higher proportion of neutralizing antibodies and Th1-biase immune response than m1Ψ-mRNA, which is more favorable to SARS-CoV-2 clearance. (E) CircRNARBD-Delta vaccine provides broad-spectrum protection against both the Delta and Omicron variants. (A), (C), and (E) were reproduced from Ref. 1.
Study findings
The current study describes the benefits and advantages of circRNA technology for COVID-19 vaccines. The circRNA vaccine platform was developed in an attempt to improve the excellent efficacy of conventional mRNA vaccines in clinical treatment.
Earlier research has suggested the use of the circRNA platform to express the desired antigen within an LNP covering. The linear RNA was converted to circular form, whereas optimizations improved transcription in vitro.
When tested in mice, the circRNA vaccine elicited high titers of immunoglobulin G (IgG) antibodies specific to the spike RBD. This serum was found to neutralize both pseudovirus and the wild-type strain of SARS-CoV-2 effectively, thereby indicating vaccine efficacy in vivo.
Further research showed that circRNA was less susceptible than linear RNA vaccines over a range of temperatures for as long as 28 days. The LNP-circRNA-RBD expressed the RBD antigen at a higher level than both the unmodified spike mRNA and optimized RNA platform containing 1mΨ, which was represented as 1mΨ-mRNARBD. The former elicited higher IgG2a and IgG2c antibody subclasses as compared to IgG1, with the higher ratios indicating Th1-skewed immune responses.
This promotes viral clearance, which is a significant benefit of circRNA vaccines. The circRNA vaccine also demonstrated its ability to induce a higher proportion of nAbs as compared to binding antibodies.
Thus, LNP-circRNARBD may be better at circumventing the antibody-dependent enhancement of infection by virus-specific antibodies.”
Three doses of the circRNARBD-Delta vaccine caused a significantly protective rise in nAbs to both the SARS-CoV-2 Delta and Omicron variants of concern (VOCs). This vaccine appears to provide broader protection against emerging SARS-CoV-2 VOCs; however, the circRNARBD-Omicron vaccine was not capable of protecting against Delta infection.
When tested in monkeys at different doses, a similar Th1-biased response was observed. When challenged with the wild-type strain of SARS-CoV-2, the viral load in these monkeys one week after infection was one thousand-fold lower. Furthermore, the lungs of vaccinated monkeys also appeared to be protected against severe COVID-19.
Conclusions
Although mRNA vaccines are highly immunogenic and able to enter the host cell, all the while resisting exonuclease digestion through many modifications of the basic structure, several limitations are associated with this vaccine platform. For example, the use of an LNP shield mandates ultracold storage and transport conditions.
The circRNA vaccines described here produce nAb titers at a similar level to those elicited by the 1mΨ-modified linear RNA vaccines. However, their covalent ring structure protects them from enzymatic degradation, thus enhancing their stability.
CircRNA vaccines can synthesize and release antigens in vivo, while inducing Th1-biased cellular immunity responses.”
Further research is needed to compare circRNA vaccines against the most commonly used mRNA COVID-19 vaccines. While the current study is not intended to present a vaccine that is ready for clinical use, its success could prompt further innovations in this field to ultimately allow for the emergence of more effective and stable mRNA vaccines.
Journal reference:
- Su, P., Zhang, L., Zhou, F., & Zhang, L. (2022). Circular RNA Vaccine, A Novel mRNA Vaccine Design Strategy For SARS-Cov-2 And Variants. MedComm. doi:10.1002/mco2.153.