
 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Background
On July 15, 2022, the European Center for Disease Prevention and Control (ECDC) categorized the SARS-CoV-2 Omicron BA.2.75 sublineage as a variant of interest (VOI). The genetic surveillance of BA.2.75 indicated that this variant was responsible for a rapid increase in the number of sequenced infections.
As compared to the Omicron BA.2 sublineage, the spike protein of BA.2.75 varied by nine amino acid residues from the N-terminal domain and the receptor-binding domain (RBD). These mutations have led to a significant reduction in viral susceptibility to neutralization by antibodies.
About the study
In the present study, researchers examine the effect of SARS-CoV-2 spike mutations on monoclonal and polyclonal antibody activity by assessing the neutralization sensitivity of SARS-CoV-2 Omicron BA.2.75.
Herein, the researchers collected serum samples from two longitudinal cohorts consisting of healthcare workers (HCWs) and elderly individuals who were eligible for Pfizer-BioNTech BNT162b2 COVID-19 vaccination.
All collected serum samples were tested to verify the absence of any antibodies that targeted the SARS-CoV-2 nucleocapsid (N) protein, as this would suggest a history of previous SARS-CoV-2 infection. Participants who had prior SARS-CoV-2 infection or tested positive on the SARS-CoV-2 nucleic acid amplification test were excluded from the study.
The researchers estimated 50% serum inhibitory dilutions (ID50s) in samples that were collected four weeks after the administration of the BNT162b2 booster vaccination in a group of 30 HCWs and elderly participants. All eligible participants had a history of previous vaccination with two doses of BNT162b2 with no incidence of intermittent SARS-CoV-2 infections.
Additionally, the activity of 17 different monoclonal antibodies that were authorized to treat SARS-CoV-2 infections was examined by estimating their 50% inhibitory concentrations (IC50s).
Study findings
Although the neutralizing activity against the SARS-CoV-2 Omicron sublineages was significantly lower than the B.1 sublineage, variations between the individual Omicron sublineages were less remarkable. The geometric mean ID50 values were 6,429, 1,725, 615, 1,052, and 1,203 against the B.1, BA.2, BA.4/5, BA.2.12.1, and BA.2.75 sublineages, respectively. As compared to other Omicron sublineages, the anti-BA.2.75 serum activity was notably lower as compared to BA.2 and higher than that against BA.4/5.
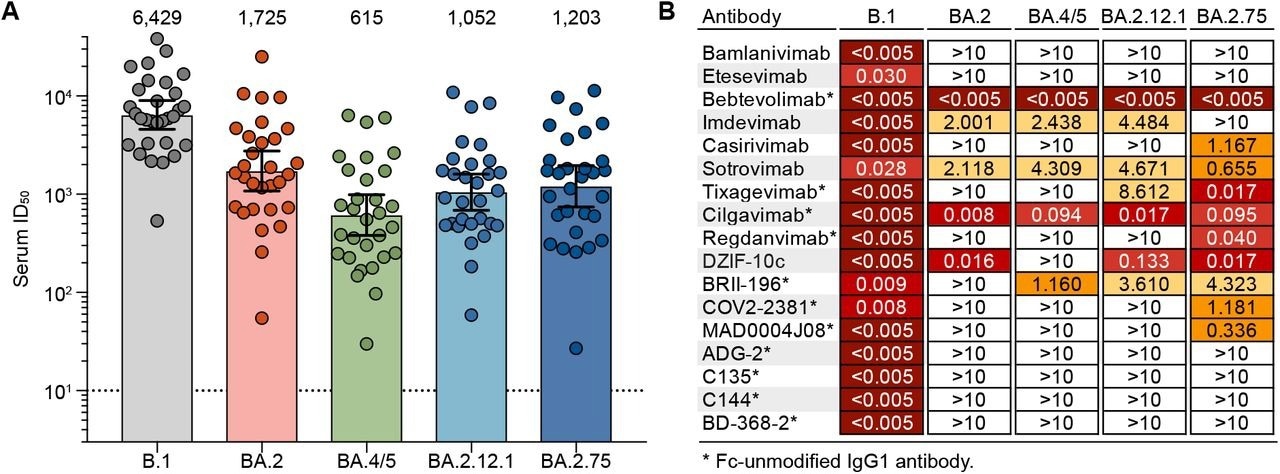 Neutralization sensitivity of the BA.2.75 sublineage. (A) Fifty-percent inhibitory dilutions (ID50s) against SARS-CoV-2 variants in samples collected four weeks after a BNT162b2 booster vaccination (n=30) determined by pseudovirus neutralization assay. Circles indicate average ID50s of two experiments for each variant and individual participant. Bars and numbers indicate geometric mean ID50s. Solid lines indicate 95% confidence intervals and the dashed line shows the lower limit of quantification. (B) Fifty-percent inhibitory concentrations (IC50s) of monoclonal antibodies against SARS-CoV-2 variants determined by pseudovirus neutralization assay (average IC50 of two experiments for each variant).
Neutralization sensitivity of the BA.2.75 sublineage. (A) Fifty-percent inhibitory dilutions (ID50s) against SARS-CoV-2 variants in samples collected four weeks after a BNT162b2 booster vaccination (n=30) determined by pseudovirus neutralization assay. Circles indicate average ID50s of two experiments for each variant and individual participant. Bars and numbers indicate geometric mean ID50s. Solid lines indicate 95% confidence intervals and the dashed line shows the lower limit of quantification. (B) Fifty-percent inhibitory concentrations (IC50s) of monoclonal antibodies against SARS-CoV-2 variants determined by pseudovirus neutralization assay (average IC50 of two experiments for each variant).
Most of the 17 monoclonal antibodies could not effectively neutralize BA.2.12.1, BA.2, or BA.4/5.; however, most of these antibodies exhibited significant activity against BA.2.75. For example, antibodies like regdanvimab and tixagevimab, which are authorized for SARS-CoV-2 treatment and prevention in South Korea, showed undetectable and poor activity, respectively, against Omicron sublineages. Nevertheless, these antibodies exhibited potent neutralization against BA.2.75.
Similarly, bebtelovimab also exhibited significantly potent neutralization activity against BA.2.75. Taken together, 30-35% of the antibodies effectively neutralized BA.2.12.1, BA.2, or BA.4/5 sublineages, while 59% of the antibodies neutralized BA.2.75.
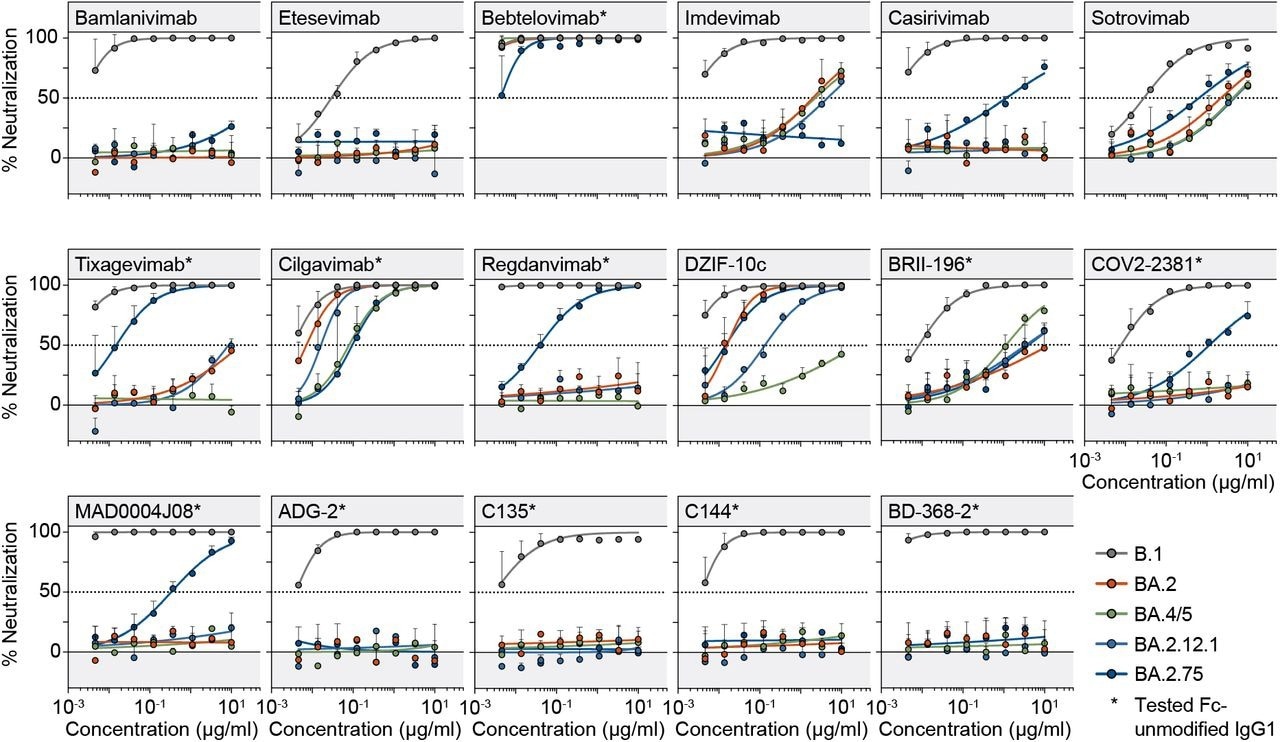 Monoclonal antibody neutralizing activity. Fitted SARS-CoV-2 pseudovirus neutralization curves of monoclonal antibodies. Circles and bars represent average values from two experiments (each performed with technical duplicates) and standard deviation. Dotted lines indicate 50% neutralization.
Monoclonal antibody neutralizing activity. Fitted SARS-CoV-2 pseudovirus neutralization curves of monoclonal antibodies. Circles and bars represent average values from two experiments (each performed with technical duplicates) and standard deviation. Dotted lines indicate 50% neutralization.
Conclusions
The study findings indicate that mutations detected in the SARS-CoV-2 Omicron BA.2.75 sublineage spike protein reduced viral susceptibility to neutralizing activity induced by COVID-19 vaccines as compared to BA.2. Moreover, BA.2.75 was more sensitive to SARS-CoV-2 neutralizing monoclonal antibodies, including those that are currently approved to treat COVID-19.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Gruell, H., Vanshylla, K., Tober-Lau, P., et al. (2022). Neutralization sensitivity of the SARS-CoV-2 Omicron BA.2.75 sublineage. bioRxiv. doi:10.1101/2022.08.04.502609. https://www.biorxiv.org/content/10.1101/2022.08.04.502609v1.
- Peer reviewed and published scientific report.
Gruell, Henning, Kanika Vanshylla, Pinkus Tober-Lau, David Hillus, Leif Erik Sander, Florian Kurth, and Florian Klein. 2022. “Neutralisation Sensitivity of the SARS-CoV-2 Omicron BA.2.75 Sublineage.” The Lancet Infectious Diseases 22 (10): 1422–23. https://doi.org/10.1016/S1473-3099(22)00580-1. https://www.thelancet.com/journals/laninf/article/PIIS1473-3099(22)00580-1/fulltext.