Introduction
Fat mass development and obesity have been associated with inappropriate food intake behaviors.
Food intake is regulated by homeostatic and non-homeostatic control systems, as well as gut microbiota-mediated signaling. While the homeostatic control pathway is triggered by hunger, non-homeostatic control pathways are associated with reward systems in the brain.
Therapeutic approaches aimed toward optimizing the gut microbiota could alter food reward perceptions and, as a result, modify food intake behaviors. These newer approaches for tackling obesity are likely to provide better success rates.
The components of the gut-brain axis
The gut-brain axis connects the central nervous system (CNS) to the GI tract to relay information regarding energy status between both systems. Both the nervous and systemic pathways are responsible for communication between the CNS and GI tract.
Some of the key components of the nervous pathway include the vagal and splanchnic nerve pathways, which allow for sensory information to travel from the upper GI tract to the CNS through afferent fibers. Some of the different stimuli from the GI tract that can be transmitted to the vagal nerve include mobility, distention, as well as the activity of different GI hormone receptors, including those for glucagon-like peptide 1 (GLIP-1), cholecystokinin (CCK), peptide YY (PYY), and ghrelin.
Conversely, the systemic pathway describes the endocrine functions of the GI tract and how they similarly transmit information regarding energy status to the brain. Intestinal enteroendocrine cells (EECs), which can be found throughout both the small and large intestines, contribute to the synthesis and release of various peptides and over 30 hormones into the bloodstream.
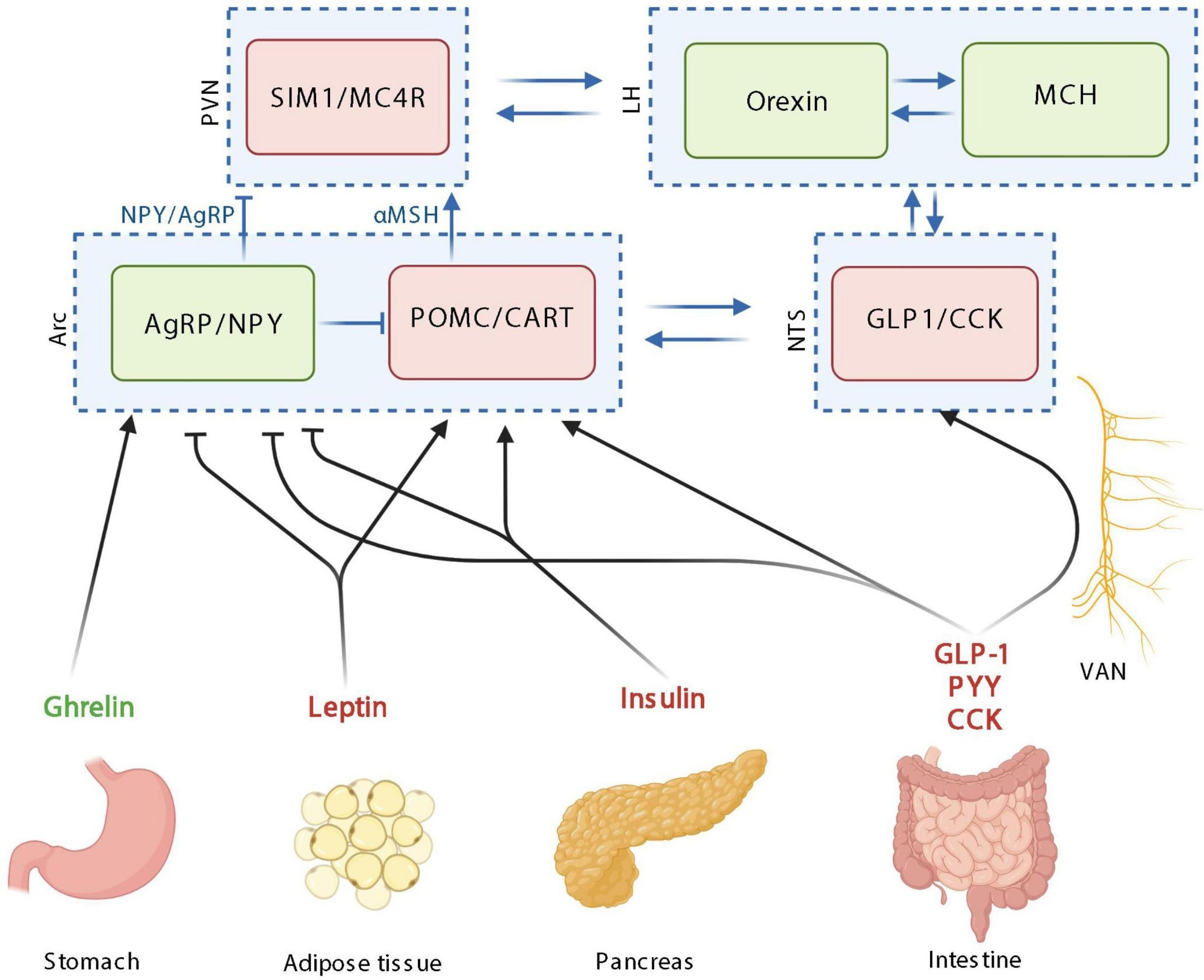 The homeostatic control of food intake upon peripheric signals. Orexigenic hormones or neurotransmitters are in green; anorexigenic hormones or neurotransmitters are in red. PVN: paraventricular nucleus; SIM1, single–minded family BHLH transcription factor 1; LH, lateral hypothalamus; MCH, melanin-concentrating hormone; Arc, arcuate nucleus; AgRP, agouti-related protein; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; CART, cocaine-amphetamine-related transcript; NTS, nucleus tractus solitarus; VAN, vagal afferent nerves; CCK, cholecystokinin; GLP-1, glucagon-like peptide 1; PYY, peptide YY; MC4R, Melanocortin 4 receptor. Created with BioRender.com.
The homeostatic control of food intake upon peripheric signals. Orexigenic hormones or neurotransmitters are in green; anorexigenic hormones or neurotransmitters are in red. PVN: paraventricular nucleus; SIM1, single–minded family BHLH transcription factor 1; LH, lateral hypothalamus; MCH, melanin-concentrating hormone; Arc, arcuate nucleus; AgRP, agouti-related protein; NPY, neuropeptide Y; POMC, pro-opiomelanocortin; CART, cocaine-amphetamine-related transcript; NTS, nucleus tractus solitarus; VAN, vagal afferent nerves; CCK, cholecystokinin; GLP-1, glucagon-like peptide 1; PYY, peptide YY; MC4R, Melanocortin 4 receptor. Created with BioRender.com.
How GI hormones control food intake
Homeostatic control of food intake monitors energy consumption to ensure that the body maintains a stable weight over time. This system relies on hypothalamic neurons to relay information to the brain regarding food intake, appetite, and satiety.
More specifically, the arcuate nucleus (Arc) in the hypothalamus consists of various types of neurons. For example, pro-opiomelanocortin (POMC) neurons suppress food intake, whereas the co-expressing Agouti-related protein (AgRP) and neuropeptide Y (NPY) neurons inhibit the actions of POMC neurons.
Insulin, CCK, GLP-1, leptin, ghrelin, PYY, and glucose-dependent insulinotropic peptide (GIP) maintain homeostatic control on food intake. Following secretion from the pancreas, insulin, for example, enters the bloodstream in response to an increase in blood sugar levels after the consumption of food. Whereas insulin is largely responsible for ensuring that peripheral tissues absorb glucose for energy purposes, this hormone inhibits the activities of AgRP/NPY neurons in the brain.
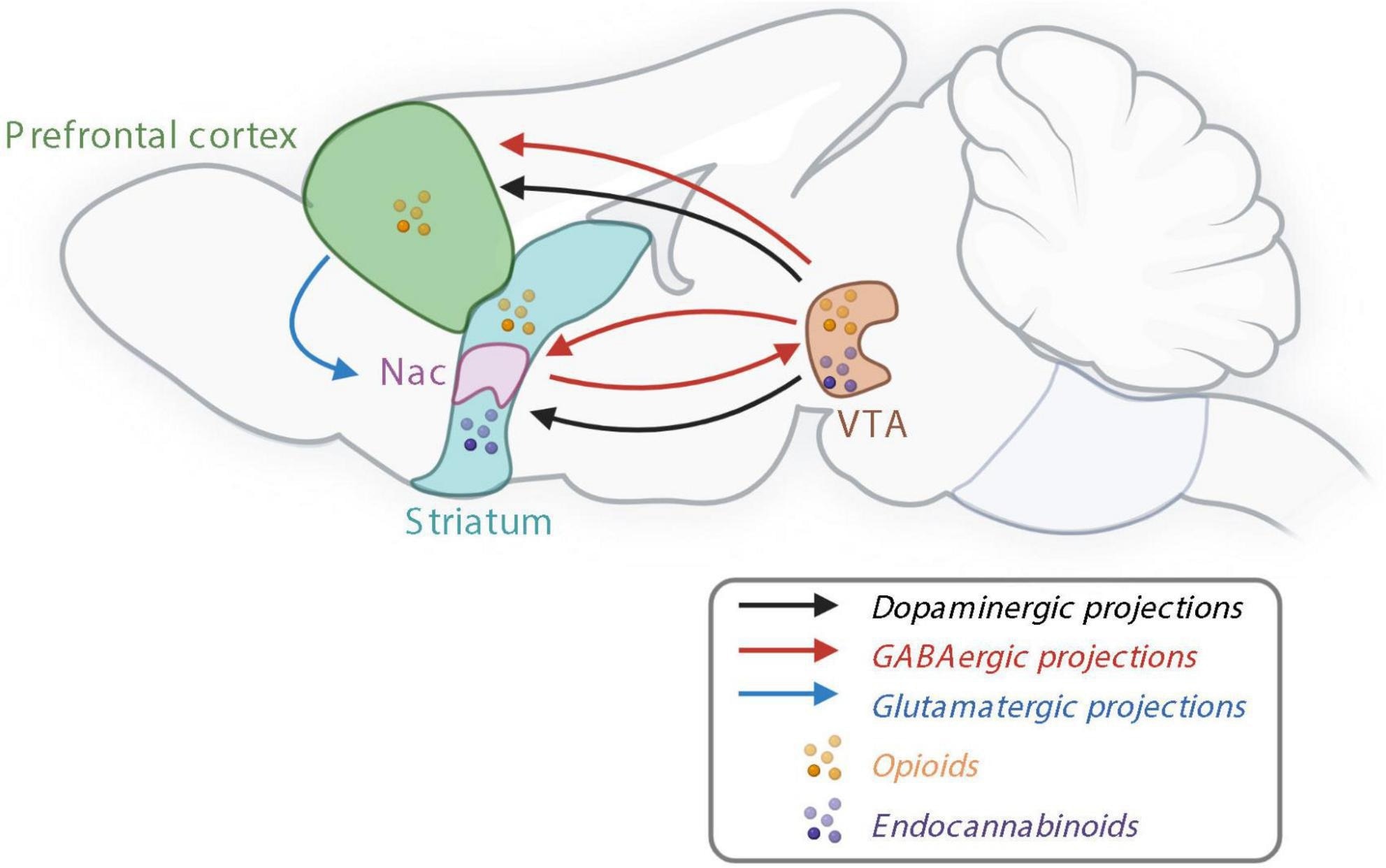 The reward system controlling non-homeostatic food intake. Nac, nucleus accumbens; GABA, γ-aminobutyric acid; VTA, ventral tegmental area. Created with BioRender.com.
The reward system controlling non-homeostatic food intake. Nac, nucleus accumbens; GABA, γ-aminobutyric acid; VTA, ventral tegmental area. Created with BioRender.com.
Leptin, which is produced by fat cells at a level that reflects the fat mass of an individual, is also released into circulation. Like insulin, leptin exerts similar effects on AgRP/NPY neurons, thereby leading to anorexigenic effects.
Conversely, ghrelin, which is synthesized by the stomach, stimulates food consumption and, as a result, is considering an orexigenic hormone. More specifically, this hormone inhibits POMC activity and activates AgRP and NPY neurons.
CCK, which is produced by a subtype of EECs, is secreted in response to certain nutrients such as lipids and proteins in the intestinal lumen. GIP is also produced in response to the presence of glucose and fats following the consumption of food. Both CCK and GIP exert anorexigenic effects, the latter of which is mediated by GLP-1.
Importantly, other organs contribute to the homeostatic control of food intake. For example, adipose tissue and the pancreas secrete adiponectin and amylin, respectively, both of which assist in controlling blood glucose levels.
The role of the reward system
Food intake is also controlled by the non-homeostatic reward system, which is responsible for the pleasurable sentiments surrounding food consumption. The reward system prefers palatable food, which is often rich in sugars and fats and is often based on the taste, odor, texture, and appearance of food.
This reward system appears to be regulated by the mesocorticolimbic pathway, which is largely controlled by dopamine, which can be released following the consumption of palatable food. Additionally, γ-aminobutyric acid (GABA) and glutamatergic releasing neurons, as well as endocannabinoids, also participate in reward processes.
Pathophysiology of obesity
Obesity is influenced by both the homeostatic and non-homeostatic systems.
Several genes are involved in the homeostatic control of food, which is often altered in morbidly obese individuals. For example, some genes appear to cause the overexpression of leptin within adipose tissues in obese individuals, which can subsequently cause leptin resistance to arise.
Insulin resistance has similarly been reported to contribute to the development of obesity and type 2 diabetes; however, this association remains poorly understood.
In terms of the impact of the reward system on obesity, long-term overeating precipitates a dysregulated dopaminergic reward system, as evidenced by reduced dopamine release.
These altered food-reward behaviors are collectively referred to as a ‘food addiction,’ as they exhibit similar neuronal behaviors to those observed during drug addiction. During food addiction, stimulation with repeated palatable stimuli will lead to a desire to increase the amount of food consumed. This will subsequently cause the individual to experience similar pleasure when eating the same food in the future.
The role of the gut microbiome
The gut microbiome modulates metabolism and food intake through the gut-brain axis. Altered composition of the gut microbiota has been proposed as a targeted approach toward obesity management, such as through the administration of commensal microorganisms like probiotics.
The absence of beneficial gut microbiota, which can arise due to the use of antibiotics, for example, has been shown to alter the dopaminergic system. In fact, germ-free mice have been shown to prefer food products that are high in lipids and sucrose as compared to control mice.
Taken together, further research involving the gut microbiota targeted approaches for treating obesity is warranted.
Journal reference:
- De Wouters d’Oplinter, A., Huwart, S. J. P., Cani, P. D., & Everard, A. (2022). Gut microbes and food reward: From the gut to the brain. Frontiers in Neuroscience. doi:10.3389/fnins.2022.947240.