Studies have indicated that SARS-CoV-2 can directly or indirectly impact the reproductive tract of men and probably make them infertile. However, information on SARS-CoV-2 presence in the semen of coronavirus disease 2019 (COVID-19) patients and associated sperm parameter alterations are limited and contradictory.
 Review: The Possible Role of SARS-CoV-2 in Male Fertility: A Narrative Review. Image Credit: WHITE MARKERS / Shutterstock
Review: The Possible Role of SARS-CoV-2 in Male Fertility: A Narrative Review. Image Credit: WHITE MARKERS / Shutterstock
About the review
In the present review, researchers summarized potential mechanisms of SARS-CoV-2-associated reproductive system impairments and probable infertility among males.
Angiotensin-converting enzyme 2 receptor, sex hormones and the testes
SARS-CoV-2 binds to the angiotensin-converting enzyme 2 (ACE2) receptor for host invasion, the expression of which is reportedly high in the testes, including Leydig cells, Sertoli cells, seminiferous tubules, and spermatogonia. Higher ACE2 positivity rates have been reported in the testes of male infertile COVID-19 patients compared to fertile patients.
Orchitis is considered a potential clinical manifestation of SARS-CoV-2 infections. The higher mortality rate among men due to COVID-19 may be due to a potential sex-dependent susceptibility resulting from the high ACE2 expression among men. Further, higher ACE2 messenger ribonucleic acid (mRNA) levels have been reported among middle-aged men than younger men.
ACE2 is a component of the RAAS (renin-angiotensin-aldosterone system), which regulates spermatogenesis, steroidogenesis, sperm function, and contractility of the epididymis. Spermatogonia with ACE2 positivity in COVID-19 patients have shown impaired spermatogenesis. In addition, SARS-CoV-2 presence with altered serum parameters has been reported in the semen of COVID-19 patients.
Lower testosterone levels, testosterone/LH ratio and follicle-stimulating (FSH)/LH ratio, and higher luteinizing hormone (LH) levels indicate primary testicular damage (especially Leydig cell damage) and have been observed in COVID-19 patients. In addition, a negative correlation has been observed between the testosterone/LH ratio and C-reactive protein (CRP) levels, the increase of which is proportional to COVID-19 severity.
Lower testosterone levels are also associated with erectile dysfunction in COVID-19 patients. The findings suggest that COVID-19 may contribute to male infertility via ACE2-mediated pathways and that infertile men may be more susceptible to COVID-19.
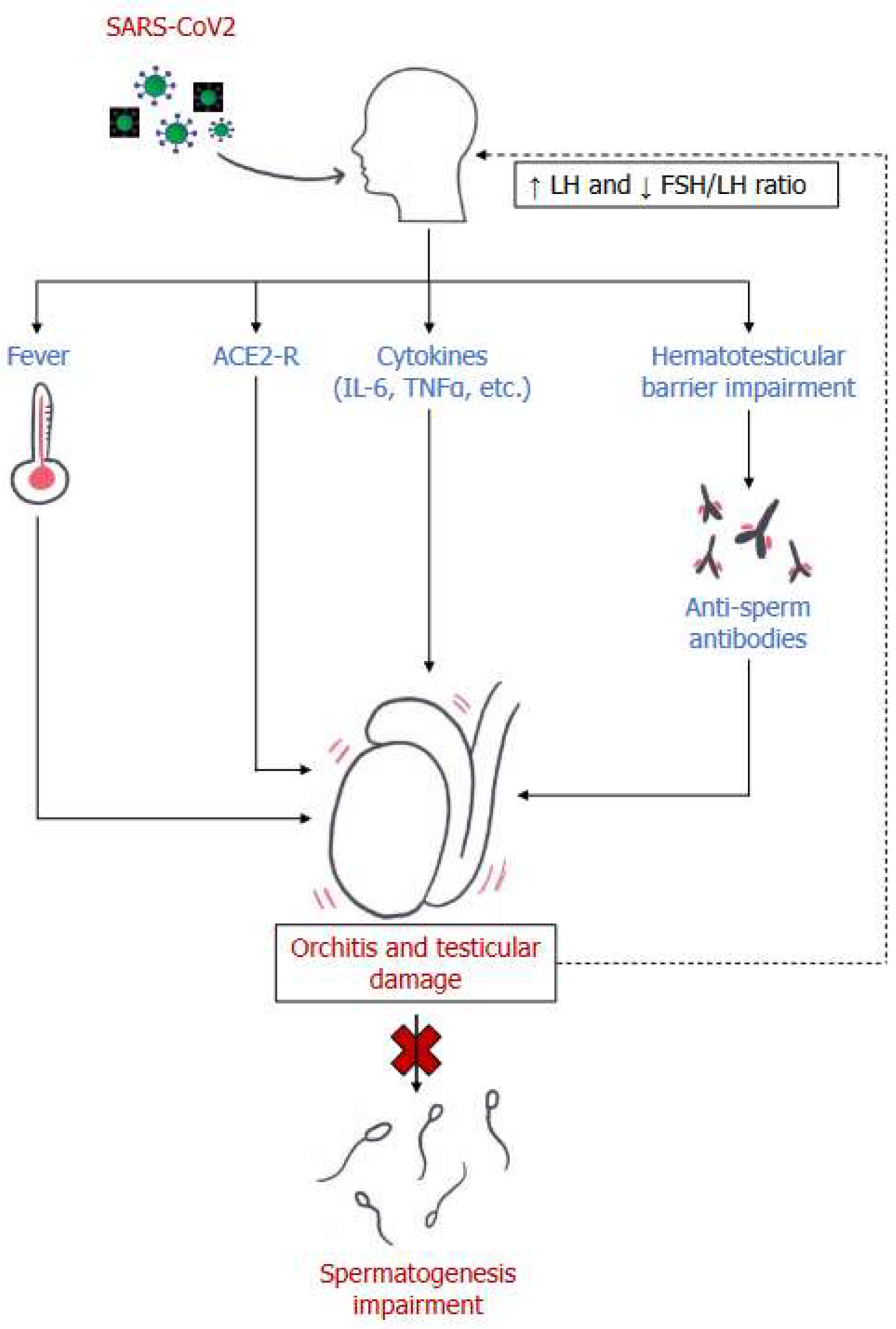 Main mechanisms of male reproductive system damage in SARS-CoV2 infection. LH= luteinizing hormone; FSH: follicle-stimulating hormone; ACE2-R= Angiotensin-converting enzyme receptor 2; IL-6= Interleukin 6; TNF-α= Tumor Necrosis Factor α.
Main mechanisms of male reproductive system damage in SARS-CoV2 infection. LH= luteinizing hormone; FSH: follicle-stimulating hormone; ACE2-R= Angiotensin-converting enzyme receptor 2; IL-6= Interleukin 6; TNF-α= Tumor Necrosis Factor α.
Orchitis and autoimmunity
Testicular pain and epididymitis-orchitis have been documented in SARS-CoV-2-positive individuals, and elevated testicular immunological responses have been indicated as causative for testicular injury and sperm parameter alterations. In addition, autopsy examinations of deceased COVID-19 patients have shown interstitial congestion, erythrocytes, and edema in epididymal and testicular tissues with high seminal interleukin 6 (IL-6) and tumor necrosis factor-alpha (TNF-α) levels.
Semen samples of COVID-19 patients have also shown increased expression of interferon (IFN)-α, IFN-γ, IL-1β,8,10, transforming growth factor beta (TGF-β), reactive oxygen species (ROS), and caspases-3, 8, and 9. The E3 ubiquitin-protein ligase, dynein regulatory complex subunit-7, and insulin-like factor-3 levels have been much lower in covid-19 patients' testes. The findings correlate with altered sperm morphology, concentration, motility, count, and semen volume in COVID-19.
Increased ROS production in COVID-19 indicates higher oxidative stress and ROS-mediated sperm functional impairments due to lipid peroxidation of sperm membranes and intracellular oxidative damage. In particular, sperm deoxyribonucleic acid (DNA) fragmentation is affected, indicative of disordered fertilization, implantation, or embryo development. In addition, electron microscopy has revealed SARS-CoV-2 presence in testes with leukocyte and macrophage infiltration.
SARS-CoV-2 infections can compromise the blood-testes barrier and favor anti-sperm autoantibody (ASA) production resulting in testicular damage. High ASA titers have been associated with low sperm motility and concentration [especially immunoglobulin A (IgA)], particularly in mild COVID-19 cases without pulmonary involvement; however, sperm parameters have shown recovery within three months. Sperm alterations and SARS-CoV-2 IgG titers against the SARS-CoV-2 spike (S) protein subunit 1 (S1) and the S1 RBD (receptor-binding domain) have correlated strongly, indicating immunological pathogenesis of reproductive tract impairments among male COVID-19 patients.
An inverse correlation between SARS-CoV-2 detection rates in semen and the timing of COVID-19 diagnosis was significantly greater among samples obtained <11 days of diagnosis. COVID-19 patients have been reported to have teratozoospermia in relation to COVID-19-associated fever; however, studies have not reported any correlation between COVID-19 severity and fever with sperm parameters. On the contrary, COVID-19 convalescents were reported as crypto-, oligo-, or azoospermic, the extent of which correlated significantly with COVID-19 severity, and oligozoospermia has been reported in fertile males.
To conclude, the review findings demonstrated that SARS-CoV-2 infections could cause male infertility; however, further research is required to improve understanding of the underlying mechanisms and the reversibility of COVID-19-associated direct and indirect testicular injury. Future studies must assess the virus's exact localization and replication dynamics in testes with comparative assessments of sperm parameters before and after COVID-19 diagnosis.