SARS-CoV-2, the causative pathogen of COVID-19, is a respiratory virus belonging to the human betacoronavirus family. The virus primarily attacks respiratory epithelial cells and induces a wide variety of symptoms, including fever, cough, fatigue, headache, dyspnea, loss of taste and smell, neurological complications, and rarely blood clotting.
Although mostly remains asymptomatic or mildly symptomatic, acute SARS-CoV-2 infection may lead to severe respiratory complications, multiorgan failure, and even death in susceptible people.
COVID-19 symptoms do not always resolve with the resolution of acute primary infection. About 30-75% of COVID-19 patients continue experiencing specific symptoms even after clinical recovery. This condition is called “long COVID.” The most common long COVID symptoms include fatigue, dyspnea, arthralgia, myalgia, cardiac complications, and memory problems.
According to the National Institute for Health and Care Excellence (NICE), UK, long COVID symptoms may persist for 4-12 weeks or in some cases, even longer. Therefore, it has been hypothesized that prolonged inflammation, viral persistence, and virus-induced autoimmune reactions could be responsible for long-term manifestations of COVID-19.
As with acute COVID-19, long-COVID symptoms are more common among the elderly and those with comorbidities. Proinflammatory cytokines were demonstrated to be predictive of long COVID in studies examining potential biomarkers during acute infection recovery. In addition, increased blood levels of urea, D-dimer, and C-reactive protein and reduced lymphocyte levels are associated with long-COVID symptoms.
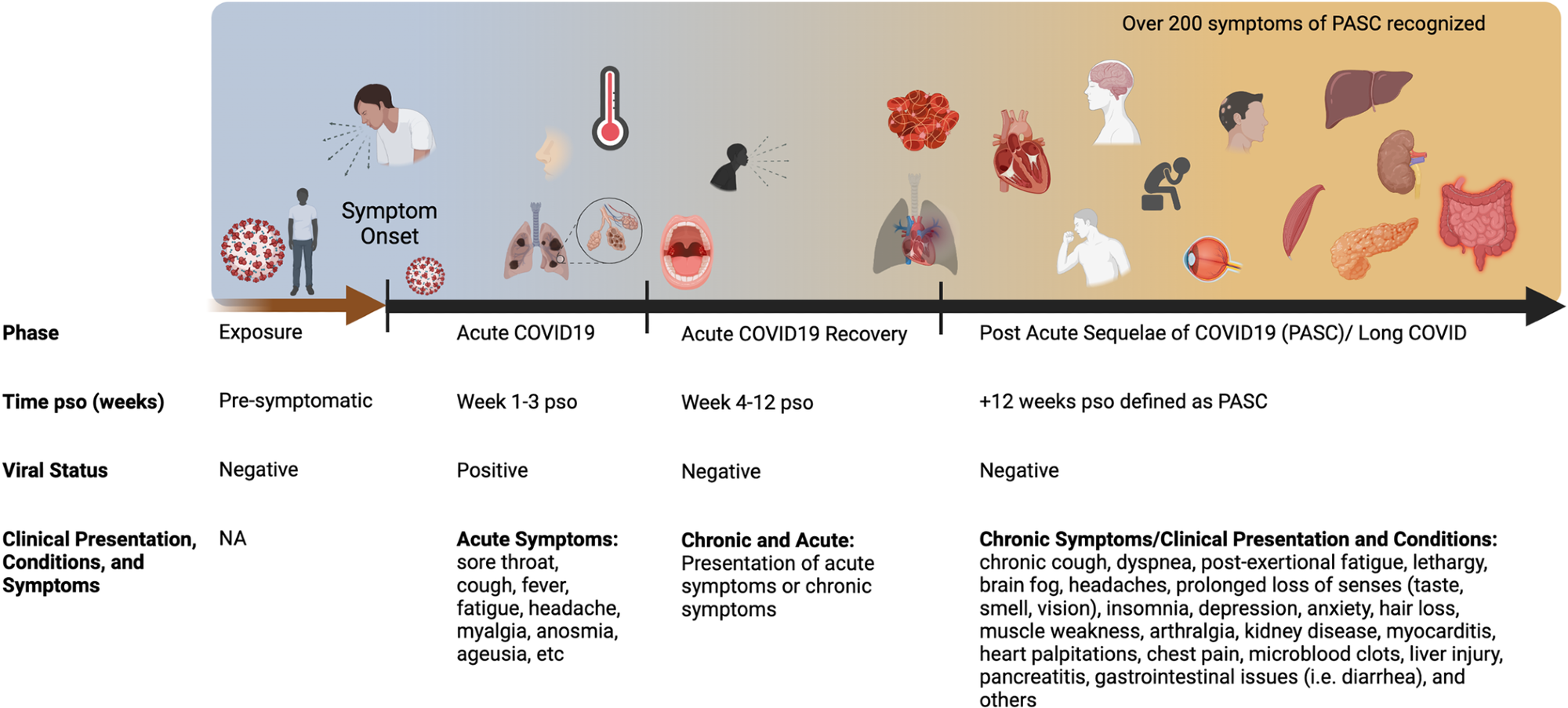 Acute COVID-19 includes symptoms present up to 4 weeks. Live virus is typically present in the respiratory tract for 1-week pso, with variability among individuals. Common symptoms of acute COVID-19 are summarized that include pulmonary and systemic manifestations. Long COVID or PASC includes persistent symptoms present 4 weeks post-acute infection. The diverse range of multiorgan complications of PASC are summarized. COVID-19, Coronavirus Disease 2019; PASC, Post-Acute Sequelae of COVID-19; pso, post-symptom onset.
Acute COVID-19 includes symptoms present up to 4 weeks. Live virus is typically present in the respiratory tract for 1-week pso, with variability among individuals. Common symptoms of acute COVID-19 are summarized that include pulmonary and systemic manifestations. Long COVID or PASC includes persistent symptoms present 4 weeks post-acute infection. The diverse range of multiorgan complications of PASC are summarized. COVID-19, Coronavirus Disease 2019; PASC, Post-Acute Sequelae of COVID-19; pso, post-symptom onset.
COVID-19 animal models to study long-COVID consequences
Several animal models, including mice, hamsters, ferrets, as well as non-human primates, have been used throughout the pandemic to study SARS-CoV-2 transmission and infection dynamics and therapeutic interventions.
Respiratory complications
Studies investigating long-COVID-related respiratory complications in hamsters have shown persistence of immune response even after systemic removal of SARS-CoV-2. A sustained infiltration of immune cells in the lungs, as well as the presence of pneumonia and lung lesions, have been observed in non-human primates after clinical COVID-19 recovery.
Since common long-COVID symptoms are known to associate with sustained immune response, these animal models could be utilized to study the etiology of prolonged respiratory complications.
On the other hand, Ferret models are not considered suitable for long-COVID studies as they develop only mild upper respiratory SARS-CoV-2 infection.
In humanized mice expressing human immune cells and human angiotensin-converting enzyme 2 (ACE2), persistent innate immune and inflammatory responses, the sustained presence of viral RNA, weight loss, and lung fibrosis have been noticed 28 days after SARS-CoV-2 infection. Thus, humanized mice could serve as a valuable model for long-COVID studies.
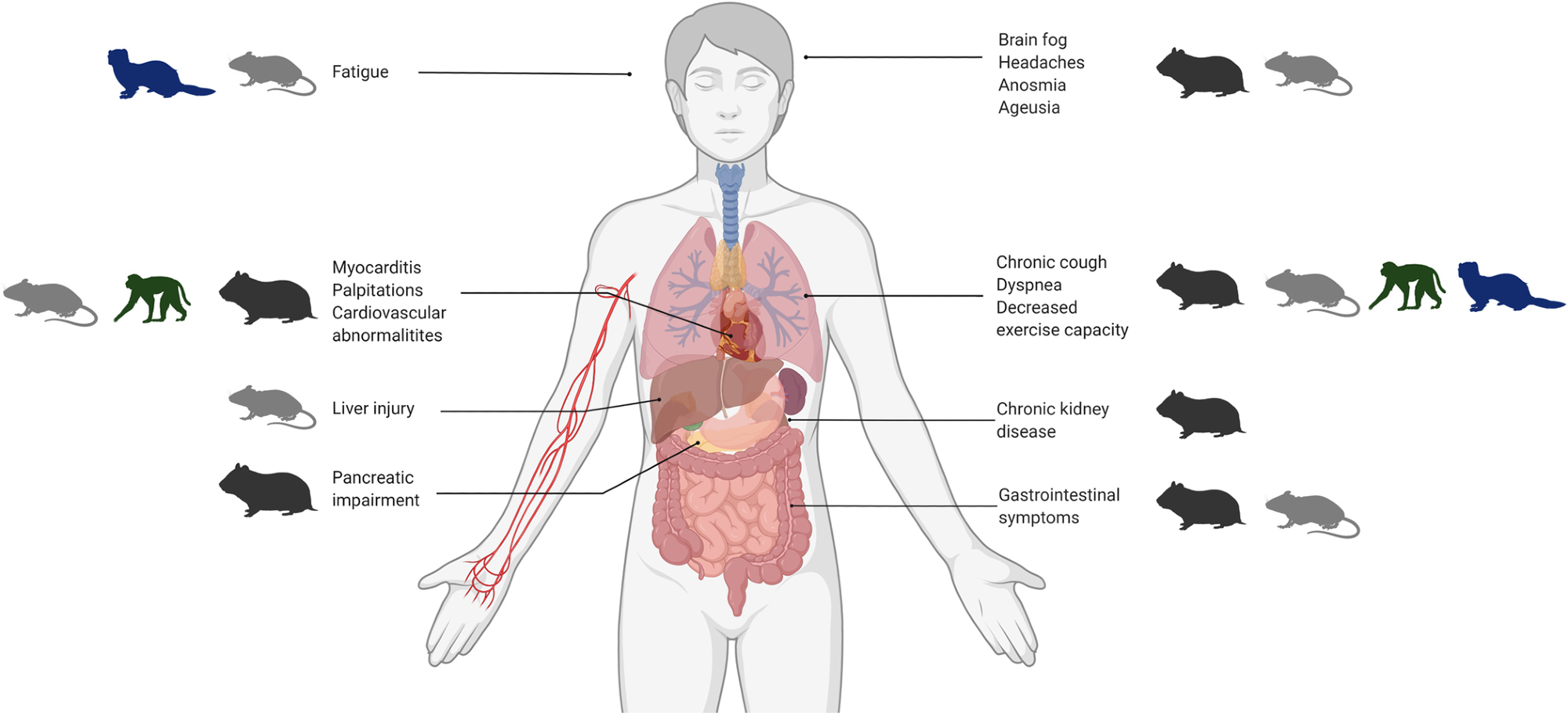 The four main groups of animal models (hamsters, mice, ferrets, and NHPs) are outlined with corresponding human manifestations of PASC. COVID-19, Coronavirus Disease 2019; NHP, nonhuman primate; PASC, Post-Acute Sequelae of COVID-19.
The four main groups of animal models (hamsters, mice, ferrets, and NHPs) are outlined with corresponding human manifestations of PASC. COVID-19, Coronavirus Disease 2019; NHP, nonhuman primate; PASC, Post-Acute Sequelae of COVID-19.
Multiorgan and systemic complications
Animal models that could be used to study multiorgan complications of long-COVID include human ACE2-expressing mice, ferrets, and hamsters. In these animals, the presence of viral RNA has been observed in multiple organs outside the respiratory tract. In hamsters, progressive bone loss has been observed 60 days after infection.
Non-human primates have been found to exhibit similar clinical manifestations and systemic immune responses as COVID-19 patients during the post-acute phase of infection. Thus, these animals could be used to study immune system-induced damages observed in long-COVID.
Cardiovascular complications
Heart palpitations, chest pains, and myocarditis are common symptoms of long-COVID, indicating the involvement of the cardiovascular system. Hamsters could serve as a valuable model to study long-COVID cardiac complications as they exhibit cardiovascular damage (ventricular wall thickening, increased ventricular mass/body mass ratio, and interstitial coronary fibrosis) up to 14 days post-infection.
Humanized mice and non-human primates could also be used to study cardiac complications as viral RNA has been detected in their heart tissues 5 – 6 weeks post-infection.
Neurological complications
Neuropsychiatric complications, including olfactory dysfunction, headaches, cognitive decline, depression, and anxiety, have been observed in long-COVID patients.
Studies investigating olfactory dysfunction in SARS-CoV-2-infected hamsters have indicated that the virus causes severe damage to olfactory epithelium during the acute infection phase. However, regular olfactory function restoration was observed soon after viral clearance.
Regarding long-term effects, a persistent disturbance in cognitive and behavioral activities has been observed in hamsters following SARS-CoV-2 infection. Overall, these observations highlight the significance of hamster models in studying long-term neurological outcomes of COVID-19.
Therapeutic interventions for long-COVID
Several clinical trials are ongoing to identify potential therapeutic interventions for long-COVID. Most of these trials investigate treatment options for respiratory complications, with some focusing on extra-respiratory manifestations.
A phase 2 clinical trial involving long-COVID patients is investigating the therapeutic efficacy of ribonuclease RSLV-132, an RNase-Fc fusion protein that prevents aberrant activation of the innate immune system. Since the persistent presence of sub-genomic viral RNA has been observed in non-human primates, they can serve as a vital model system to study the therapeutic efficacy of ribonuclease RSLV-132.
Sodium pyruvate, which has been found to reduce long-COVID symptoms in patients, could be tested for detailed therapeutic characterization in hamsters because of their similarities with humans in exhibiting long-COVID multiorgan symptoms.
Overall, these observations highlight the significance of different animal models in exploring long-COVID pathogenesis and therapeutic interventions.