CoVs, particularly beta-CoVs, thrive in animal reservoirs and constantly threaten human health. Seven CoVs infect humans, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and four circulate endemically. Zoonotic spillover of beta-CoVs into humans is associated with elevated disease morbidity and mortality, economic burden, and epidemics.
SARS-CoV-2 evolution will likely continue, and the virus may become the next endemic human CoV (HCoV). SARS-CoV-2 variants of concern (VOCs), such as Delta and Omicron, highlight the threat of escape from vaccine-induced antibodies and the pressing need for creating vaccines that induce broadly protective immune responses against different CoVs.
 Study: Nanoparticle display of prefusion coronavirus spike elicits S1-focused cross-reactive protection across divergent subgroups. Image Credit: Orpheus FX / Shutterstock
Study: Nanoparticle display of prefusion coronavirus spike elicits S1-focused cross-reactive protection across divergent subgroups. Image Credit: Orpheus FX / Shutterstock

 *Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
The study and findings
In the present study, researchers engineered nanoparticles displaying the spike protein trimers from different CoVs. The two proline mutations (2P) which stabilize the prefusion spike conformation were introduced in the spikes from SARS-CoV, Middle-east respiratory syndrome (MERS)-CoV, and SARS-CoV-2, and adapted the antigens for display on the previously-described two-component nanoparticle platform, I53_dn5.
The prefusion-stabilized spike (S2P) antigens were fused to the trimeric component (I53_dn5B) and assembled by adding the pentameric component (I53_dn5A) in vitro to create monotypic particles expressing 20 copies of the S2P trimer. The antigenicity of purified nanoparticles and S2P trimers was tested by enzyme-linked immunosorbent assay (ELISA) using monoclonal antibodies (mAbs) against each CoV spike.
Antibody binding was comparable between S2P and nanoparticles, implying a similarly intact antigenicity in each nanoparticle formulation. Next, the authors investigated whether the multivalent display of S2P trimers induced a greater antibody response than soluble S2P. To this end, BALB/c mice were immunized twice with the soluble form of SARS-1_S2P or as a nanoparticle formulation displayed on I53_dn5 and MERS_S2P or influenza hemagglutinin (HA) expressed on I53_dn5.
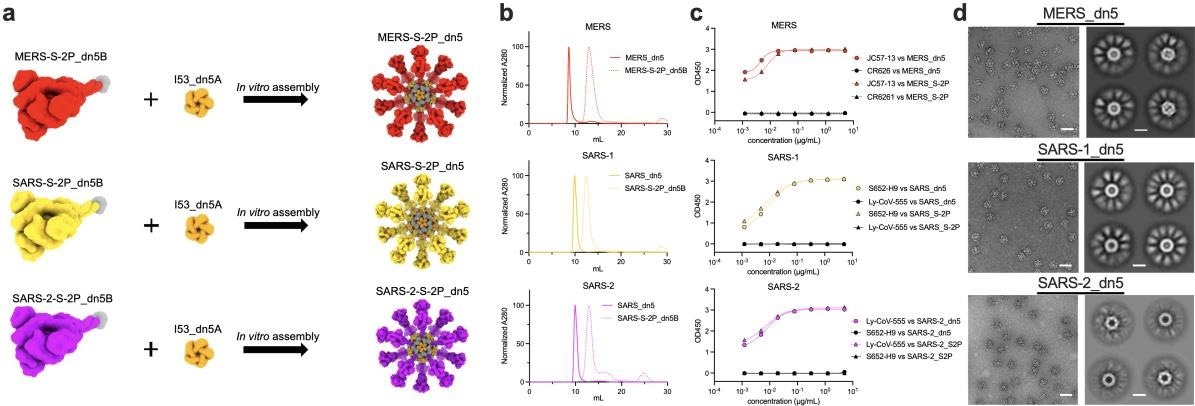
Design and characterization of CoV-S-2P displayed on I53-dn5. a Computer-generated models of prefusion-stabilized spike trimers (S-2P) from MERS, SARS- 1, and SARS-2, and their homotypic display on I53-dn5 nanoparticle. Icosahedral nanocage displays 20 trimers. b Trace profiles of S-2P_dn5B trimer and _dn5 nanocage purification by size exclusion chromatography. c Antigenicity ELISA comparing binding of antibodies specific for MERS, SARS-1, or SARS-2_S-2P to soluble trimer (triangles) or dn5 assembly (circles) respectively. d Representative images of CoV-S-2P_dn5 at 50,000x magnification and 2D class averages. Scale bars correspond to 100 nm (representative images) and 20 nm (2D class averages).
Serologic responses were evaluated after five weeks for cross-reactive immunoglobulin G (IgG) antibodies. Immunization with soluble SARS-1_S2P elicited higher antibody titers against S2P trimers of SARS-CoV, MERS-CoV, and SARS-CoV-2 than with SARS-1_I53_dn5. Antibodies elicited by soluble S2P and nanoparticle-displayed S2P neutralized SARS-CoV pseudoviruses, but not SARS-CoV-2. Notably, antibodies generated by SARS-1_I53_dn5 neutralized MERS-CoV pseudovirus at similar levels as MERS_I53_dn5.
Further, the team noted that the assembly of SARS-1_S2P on I53_dn5 restricted access of the B-cell receptor to the spike’s S2 domain, resulting in B cell selection and antibody responses to the S1 domain. In addition, the authors profiled the breadth of cross-reactivity of antibodies induced by additional beta-CoV S2P antigens on I53_dn5 and a mosaic nanoparticle co-displaying each of the beta-CoV S2P antigens.
BALB/c mice immunized with beta-CoV mosaic nanoparticles co-displaying S2P of SARS-CoV, MERS-CoV, SARS-CoV-2, HCoV-HKU1, and HCoV_OC43 had high antibody titers comparable to their corresponding monotypic I53_dn5 counterparts. Moreover, the mosaic nanoparticle induced cross-binding antibodies to an alpha-CoV, HCoV-229E.
Further, 288/330 +/+ mice were immunized with SARS-1_I53_dn5, SARS-2_I53_dn5, MERS-I53_dn5, or the beta-CoV mosaic nanoparticle and challenged with a lethal dose of mouse-adapted MERS-CoV strain. SARS-1_I53_dn5 nanoparticle elicited cross-subgroup immunity at levels approaching those of MERS_I53_dn5. Animal lungs were harvested five days after the lethal challenge.
The lung discoloration score of mice immunized with the beta-CoV mosaic nanoparticle was zero for three (out of four) tested mice. Lung viral titers of mice immunized with the mosaic nanoparticle were low at similar levels as those immunized with MERS_I53_dn5. In addition, lung discoloration scores of those immunized with SARS-1_I53_dn5 and SARS-2_I53_dn5 were relatively lower than those immunized with their corresponding soluble S2P trimers.
Conclusions
In summary, the authors adapted the I53_dn5 nanoparticle platform to co-display S2P antigens from diverse CoVs. They demonstrated that the quality and breadth of cross-reactive antibodies induced by the monotypic display of CoV S2P trimers were extensive. Nonetheless, the mosaic presentation of CoV S2P antigens on I53_dn5 can yield similarly potent antibody responses as their monotypic counterparts, despite the lower molarity of S2P immunogens from each CoV.
Notably, there was a lack of antibodies against the more conserved S2 domain using SARS-1_I53_dn5 as the immunogen due to the restriction of BCR access to the S2 domain. The monotypic nanoparticle immunogens elicited distinct cross-reactive antibody profiles against each HCoV. Both SARS-1_I53_dn5 and SARS-2_I53_dn5 immunogens induced similar cross-reactive antibodies and MERS_S2P-reactive antibodies.
However, immunization with SARS-1_I53_dn5 only protected mice from the lethal challenge of MERS-CoV. Typically, neutralizing potency decreases with the increase in the breadth of responses, underscoring the significance of understanding orthogonal antibody functions. Therefore, future research must determine the optimal mosaic nanoparticle design to confer maximum breadth and potency.

 *Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: Research Square publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.