Before participating in the research on the global burden of antimicrobial resistance (AMR), as a medical doctor, clinical microbiologist, and biomedical scientist, I was a part of relevant research endeavors on antibiotic resistance in my home country Croatia – such as a nationwide study on extended-spectrum beta-lactamases and plasmid diversity in urinary Escherichia coli isolates, as well as describing the emergence of multidrug-resistant Proteus mirabilis in long-term care facilities.
Since my Ph.D. thesis was on addressing AMR in the most common sexually transmitted bacterial agent, Chlamydia trachomatis, I was also a part of the team that aimed to standardize the method for laboratory susceptibility testing of chlamydiae by using both special cell culture and direct molecular-based monitoring, which was published in one methodological textbook.
Therefore, I would say AMR in different microorganisms was always my passion – from the diagnostic standpoint and the therapeutic one. More specifically, I was also involved in certain aspects of drug research, such as proposing a novel dual antagonist to prevent and treat urinary Escherichia coli infections and the usage of liposomal encapsulation to increase the efficacy of azithromycin against Chlamydia trachomatis. The latter technology gained a lot of prominence when liposome-based mRNA COVID-19 vaccines entered the market, so it is no wonder that we tried to capitalize on the positive aspects of such an approach.

Regarding my other professional positions, I am also a Secretary General of the Croatian Society for Clinical Microbiology, Executive Committee Member of the ESCMID study group for Mycoplasma and Chlamydia Infections, and External Affairs Committee Member of the Society for Healthcare Epidemiology of America (SHEA). I have several leadership roles in the American Society for Microbiology (ASM), where I organized conference sessions on antibiotic resistance, such as the Track Hub Session for ASM Microbe, “The global scenario of antimicrobial resistance: do developing and developed countries share the same threats?”. Finally, I am very invested in science communication. As one of the writers for News-Medical, I have written several pieces on the topic of antimicrobial resistance and many other topics.
AMR is a threat to not only humans but also animals, plants, and the environment. Can you tell us more about what exactly AMR is?
Antimicrobial resistance (AMR) is regarded as one of the predominant and most salient public health issues of the 21st century, as it threatens the effective treatment and prevention of an ever-growing range of infections caused by bacteria, viruses, fungi, and parasites. In other words, these groups of microorganisms are no longer susceptible to the common medical agents used to treat them, and the issue is particularly serious and urgent in bacteria. This is an evolving issue that took place over several decades, resulting in frequent pathogenic bacteria harboring some type of resistance to each new antibiotic coming to the market. This means there is an urgent call for action to avoid a global crisis in health care when we can lose the ability to perform surgeries and other types of quotidian medical procedures.
In an attempt to define AMR, we can say that this is a natural phenomenon arising when microorganisms are exposed to antimicrobials or antibiotics. Under such selective pressure, susceptible bacteria are inhibited or killed, whereas those that are naturally (or intrinsically) resistant or those with antibiotic-resistant traits have a much greater chance of surviving and multiplying. The issue arises not only as a result of the overuse of antimicrobial agents but also when they are used inappropriately (such as inadequate drug choices, faulty dosing regimens, and/or low compliance to relevant treatment guidelines). All of this can have a compounding effect and contribute to the rise of antibiotic resistance.
During the last few years, the importance of animal reservoirs and the environment in spreading AMR has been widely recognized. In the past several decades, we have witnessed an increased awareness of the potential problems that resistance among food-producing animals could have on human health. In addition, the soil is regarded as a reservoir of AMR genes since most antibiotics are derived from soil microorganisms that are intrinsically resistant to the antibiotics produced. Finally, water potentially contaminated with organic fertilizers and fecal microorganisms may disseminate resistant bacteria in the soil and is considered a principal way of bacterial propagation between various environmental compartments.
Given the dangers of AMR and the slogan of World Antimicrobial Awareness Week - ‘Antimicrobials: Handle with Care,’ why is it crucial to handle antimicrobials with care?
Judicious and careful use of antimicrobial agents is one of the pillars of successfully diminishing the threat of AMR. In the clinical milieu, there is an important concept of antimicrobial stewardship that refers to a set of coordinated strategies for improving patient care and outcomes by instituting optimal therapy, minimizing collateral damage by reducing antimicrobial usage (which translates to lower resistance rates), and lowering the price of antimicrobials. This concept is also amenable to global implementation to help control AMR by increasing awareness of the public and educating healthcare professionals on the prudent use of antimicrobials.
In the hospital setting, antimicrobial stewardship programs and infection control measures are of utmost importance to prevent the emergence and transmission of antibiotic-resistance microorganisms and preserve the effectiveness of currently available antimicrobial drugs. Hence, multidisciplinary teams of experts (such as infectious disease specialists, medical microbiologists, and clinical pharmacists) participate in such endeavors. Moreover, as the ongoing COVID-19 pandemic can lead to the increased indiscriminate usage of antimicrobials (which was particularly the case in the early days of SARS-CoV-2 spread), handling antibiotics with care can result in lower bacterial resistance and, subsequently, a lower death toll.
Nevertheless, the antimicrobial stewardship concept has to be extended to family doctors in the community, where there is often a very high consumption of antibiotics. Relevant public health actions that are needed to reduce inappropriate antimicrobial prescriptions and antibiotic misuse should consider adequate information campaigns for the consumers, training of healthcare professionals, enhanced diagnostics to improve treatment decisions, the development of treatment guidelines, as well as regular prescription audits. In a nutshell, different healthcare organizations should strive to make coordinated efforts to institute new policies and put more emphasis on antimicrobial stewardship in professional curricula.
 Image Credit: dturphoto/Shutterstock
Image Credit: dturphoto/Shutterstock
You recently published research concerning the burden of bacterial antimicrobial resistance in the WHO European region. Can you tell us more about this study and the results you identified?
To our knowledge, this new study brings the most comprehensive analysis of the AMR burden in the WHO European region, and our estimates span across 53 countries, 23 bacterial pathogens, and 88 pathogen–drug combinations in 2019. There are several advances in comparison to previous work on this topic, primarily in scope (as not only the European Union is included, but all countries of the WHO European region), as well as in the number of included pathogen-drug combinations.
Furthermore, we used major methodological innovations that were first identified in the 2019 global burden of bacterial AMR study. The magnitude of the problem was described with the use of two scenarios, which means we provided estimates for both deaths directly caused by AMR (attributable mortality) and deaths that occurred from a drug-resistant infection, but for which AMR may or may not have been the cause (associated mortality).
Finally, our study allows comparisons with other causes of death since it builds on estimates of disease incidence, prevalence, and mortality from the Global Burden of Diseases, Injuries, and Risk Factors Study 2019.
And results were striking. By identifying more than half a million deaths associated with AMR and more than 130 thousand deaths attributable to AMR, we have shown that antibiotic resistance is a considerable and potentially neglected problem in the WHO European region as a whole, with evident differences between subregions and specific countries. The largest fatal burden of AMR in the region came from bloodstream infections, followed by intra-abdominal infections and respiratory infections. The leading pathogens that we identified were (in descending order of death) Escherichia coli, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa and Enterococcus faecium.
Such estimates of the impact of AMR on morbidity and mortality are crucial for informing public health investment decisions for each country in this region. Furthermore, highlighting specific pathogens and pathogen–drug combinations with the highest estimated burden – which we showed were methicillin-resistant Staphylococcus aureus (MRSA) and aminopenicillin-resistant Escherichia coli – might specifically inform policy targets and policy design. Our results emphasize that the most effective way to address AMR in this region will necessitate targeted efforts and investments, together with continuous outcome-based research endeavors.
 Study: fizkes/Shutterstock
Study: fizkes/Shutterstock
What do you believe are some of the challenges with approximating the magnitude of the AMR crisis and its downstream effect on human health?
There are indeed many challenges with this type of complex estimation process, and the biggest one is definitely data scarcity, as the availability of data on AMR can differ from one country to the next. This is not the only problem with our study but is universal for all research projects that aim to assess the burden of AMR. We also acknowledge that the effect of resistance on mortality may differ across locations, which can be pertinent when we pursue a global estimation of the AMR crisis.
More specifically, certain locations might not be well-suited to treat susceptible infections, which means that the effect of resistance is minimized; conversely, other locations might not have access to second-line antimicrobials; thus, the effect of resistance is magnified. It is also possible that the relative risk of death attributable to resistance can be different across anatomical sites of infection due to variable antibiotic penetrance.
Moreover, countries with low socio-demographic index (which is a summary measure that combines information on the education, economy, and fertility rate) might have much less stringent surveillance systems, as well as insufficient laboratory support – potentially resulting in an underestimation of attributable and associated AMR mortality globally and the countries of the WHO European Region. Nonetheless, our estimates are informed by data from all countries included in the study. When data for a specific country were lacking, estimates and model building relied on regional patterns, co-variates, and out-of-sample predictive validity.
Despite these limitations, our analysis reflects the widest and presently best available range of data, as well as the use of models that have been developed and implemented specifically for incorporating disparate data sources for the Global Burden of Disease analysis. We are in agreement with other studies that highlight and underscore critical data gaps on resistant organisms in certain parts of the world; therefore, solving this problem which will be extremely important in the future to fine-tune our estimates additionally.
WHO: What is antimicrobial resistance (AMR)?
The specific theme of World Antimicrobial Awareness Week (WAAW) 2022 is ‘Preventing antimicrobial resistance together.’ What does this theme mean to you personally, and how do you believe we can take steps toward this goal?
It is without any doubt that manifold joint efforts from healthcare workers (acting as prescribers) and patients to policymakers and international regulators are necessary to stand a chance against the global spread of antibiotic resistance. In other words, different stakeholders have to join forces in order to tackle this issue from many angles, as no single action will provide an acceptable solution in isolation.
Also, this issue is a truly global problem. Together with rational and prudent usage of currently available antimicrobial drugs and the introduction of antibiotics where there is a lack of them, the development of new and effective compounds, as well as the introduction of new diagnostic approaches, are all recognized as urgent priorities.
Governments should introduce several essential processes to inspire change by all stakeholders related to AMR, as appropriately described within the WHO policy package for combating drug resistance. More specifically, this policy package refers to a national plan that strives to be comprehensive, engages civil societies, and insists on the accountability of everyone involved. Also, strengthened surveillance systems, improved laboratory capacity, wide access to essential medicines of sufficient quality, regulated use of antibiotics, the emphasis on infection prevention and control, as well as promotion of innovations will be crucial in the near future. There has to be a commitment to a rather high level of human health protection.
How do you believe that different sectors, for example, healthcare, animal care, farming, and agriculture, can work collaboratively to help curb AMR?
Our quest against AMR should be addressed through the lens of a One Health approach. This means more stringent infection prevention/control in healthcare facilities, food industry premises, and farms, as well as insisting on best practices in agriculture, clean water, sanitation and waste management. A set of diverse but coordinated strategies against antibiotic resistance should be implemented, taking into account the type of pathogen (either human or zoonotic), the setting (healthcare or the community) and possibly other specific factors contributing to the emergence of resistance.
In veterinary medicine, the required interventions consist in enforcing regulations for improved surveillance and monitoring, governing the use of antimicrobials in food-producing animals, and decreasing the need for antibiotics through improved animal husbandry. Naturally, more research is needed to elucidate the exact pathways of transmission of resistant microbial agents between animals and humans (but also their subsequent impact). There is a need to adequately implement legislation if we are to achieve long-lasting effects.
In addition, innovative approaches are needed for the development of new antibiotics and other products to limit AMR. There is a shortage of new antibiotics in the pipeline and few incentives for the industry to invest in research and development in this field. Research into digital technologies and eHealth solutions has to be strengthened to improve prescription practices, care solutions, and overall awareness of this issue. All of this necessitates a well-designed roadmap to orchestrate further collaboration efforts between governments, industry, and non-governmental organizations.
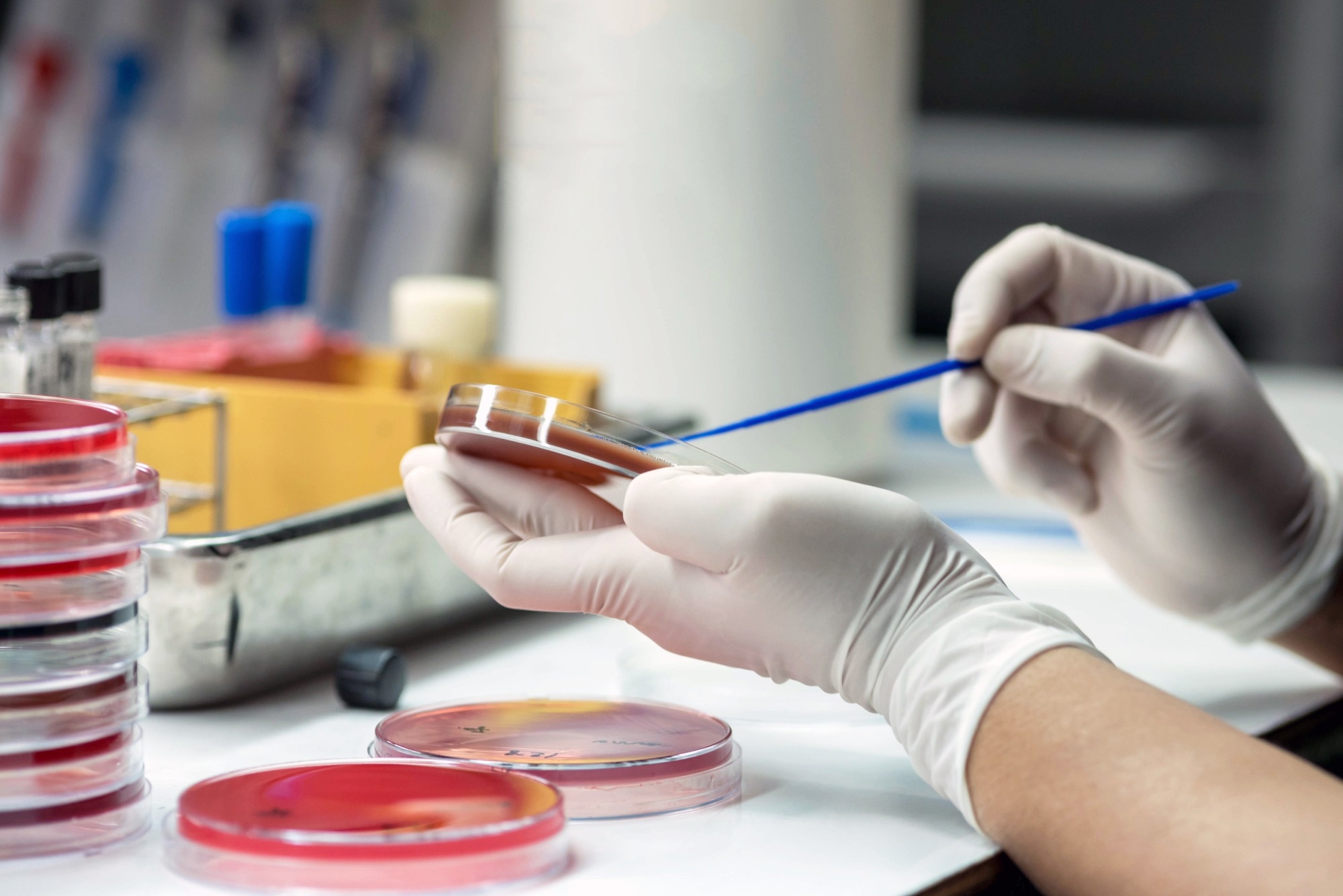 Image Credit: AnaLysiSStudiO/Shutterstock
Image Credit: AnaLysiSStudiO/Shutterstock
What are the next steps for you and your research? Do you have any exciting projects coming up?
The Global Research on AntiMicrobial resistance (GRAM) Project will definitely continue to be one of the most important global projects in years to come. Assessing the burden of bacterial antimicrobial resistance in the WHO European region in 2019 was our first regional endeavor, which will be followed by research publications covering other regions of the world. We believe it is of utmost importance to obtain a full picture of this pressing issue not only on a global but also on a regional and country level. In the future, one of the goals is to pursue a time-series analysis of the AMR burden through the years, which will be helpful in forecasting, preparedness planning, and key policy decisions.
Furthermore, we have already mentioned how the animal and environmental sectors present a plethora of opportunities for resistance to evolve and be introduced into human populations. Therefore, we believe it will be important to assess data gaps and links between animal and human resistance in one of our future projects. Our goal is also to assess significant indirect effects of AMR, such as the effect of AMR on antibiotic prophylaxis in transplant recipients or for the prevention of surgical site infections. One of the salient goals is to assess AMR in the context of health equity, particularly considering the results from the paper on the global burden of AMR.
Finally, there is a need to prioritize the improved collection of high-quality AMR data in both the human and animal sectors, as well as the environment, in order to improve all our future estimation processes. One of our goals is to facilitate data and resource sharing between countries to improve policy-making and capacity building. Finally, continuously broadening both the quantity and quality of data acquisition worldwide will allow us to monitor levels of resistance much more effectively and course-correct action where needed. We are confident that our data-driven approach will result in even more stringent estimates and help in tackling this enormous challenge.
Where can readers find more information?
About Dr. Tomislav Meštrović
Dr. Tomislav Meštrović is an Associate Professor at the University North in Croatia and an Affiliate Associate Professor at the Institute for Health Metrics and Evaluation (IHME) and the Department of Health Metrics Sciences of the University of Washington. He finished his medical and doctoral training at the University of Zagreb School of Medicine (Croatia), his MPH at the London School of Hygiene and Tropical medicine of the University of London (United Kingdom), and his MBA in International Healthcare Management at the Frankfurt School of Finance & Management (Germany). He is a board-certified clinical microbiology and sexual medicine specialist, with an additional one-year training in clinical research from Harvard Medical School.
Metrics Sciences of the University of Washington. He finished his medical and doctoral training at the University of Zagreb School of Medicine (Croatia), his MPH at the London School of Hygiene and Tropical medicine of the University of London (United Kingdom), and his MBA in International Healthcare Management at the Frankfurt School of Finance & Management (Germany). He is a board-certified clinical microbiology and sexual medicine specialist, with an additional one-year training in clinical research from Harvard Medical School.
His primary research interest with IHME is the public health significance and impact of antimicrobial resistance (AMR) within the Global Burden of Antimicrobial Resistance (GRAM) project, working in the AMR research team led by Professor Mohsen Naghavi. He joined this group as a Fulbright Visiting Scholar during the academic year 2021/2022, and was a lead author on the comprehensive assessment of AMR burden in the WHO European Region. Alongside his ongoing work in antibiotic resistance, he participates in other IHME-led and GBD-related projects, providing expertise for many pivotal global and public health research questions (particularly those in relation to infectious diseases). He is also a member of the WHO/HIFA Working Group Member on Learning for Quality Health Services, which is a part of the WHO Global Learning Laboratory (GLL) for Quality Universal Health Coverage (UHC).
IHME was established at the University of Washington in Seattle in 2007. Its mission is to deliver to the world timely, relevant, and scientifically valid evidence to improve health policy and practice.