Although the gut microbiome remains stable in the long term, diet can induce transient changes in its composition. Similarly, the gut microbiome shows rhythmic variations in composition and localization throughout a day-night cycle.
In the current study, scientists have explored the interplay between the gut microbiome, time-restricted feeding pattern, and circadian rhythms in the Drosophila (common fruit fly) gut.
Circadian regulation of gut microbiome
Drosophila with an unlimited food supply (ad libitum) were used to study whether the gut microbiome composition and diversity change over the course of the day-night cycle. The findings revealed no circadian cycling of the gut microbiota in wild-type flies and in flies lacking a circadian clock gene.
The wild-type flies subjected to rhythmic feeding patterns at specific time points also revealed no circadian variations in gut microbiota composition and diversity. However, in flies lacking a circadian clock gene, circadian clocks were found to interfere with the effect of time-restricted feeding patterns on the gut microbiome.
Impact of time-restricted feeding on circadian transcriptome
The analysis of circadian gene expression was conducted in the study using flies with and without a microbiome. The findings revealed that both time-restricted feeding and microbiome loss significantly impact the gut's circadian transcriptome.
Host genes encoding metabolic proteins showed increased cycling in flies without a microbiome (sterile). In contrast, genes associated with development and differentiation showed reduced cycling.
In flies maintained under time-restricted feeding conditions (food provided at specific time points), loss of cycling expression was observed for genes associated with oxidative phosphorylation and energy metabolism.
An induction in cycling gene expression was observed under time-restricted feeding conditions. Altered activities of transcription factors were found to be responsible for the global changes in cycling expression. Similar to time-restricted feeding, loss of microbiome was found to influence cycling gene expression.
The effect of time-restricted feeding on cycling gene expression appeared to be mediated by increased histone acetylation. The findings revealed that restricted feeding conditions trigger robust rhythms of histone acetylation and that many genes affected by these conditions are responsive to histone acetylation.
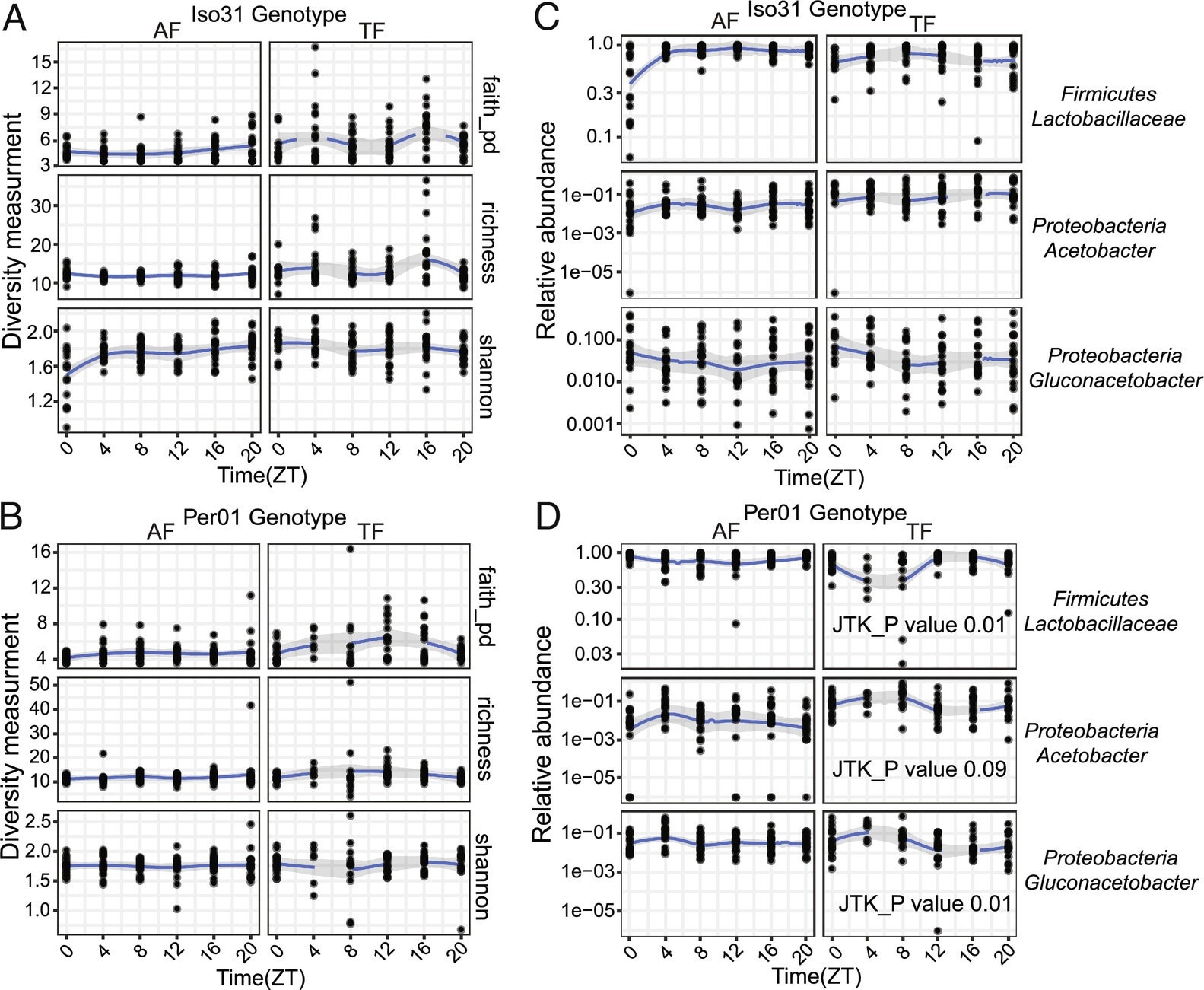 The Drosophila intestinal microbiome is largely stable over a daily cycle. (A and B) Microbiome diversity does not show diurnal oscillations in wild type Iso31 or clock mutant per01 fly guts under ad−lib (AF) or TF conditions. JTK_cycle was used to assess rhythmicity. (C and D) Specific bacterial species cycle under TF conditions, but only in per01. JTK_cycle values are shown.
The Drosophila intestinal microbiome is largely stable over a daily cycle. (A and B) Microbiome diversity does not show diurnal oscillations in wild type Iso31 or clock mutant per01 fly guts under ad−lib (AF) or TF conditions. JTK_cycle was used to assess rhythmicity. (C and D) Specific bacterial species cycle under TF conditions, but only in per01. JTK_cycle values are shown.
Functional impact of time-restricted feeding and microbiome
Flies maintained with time-restricted feeding were subjected to different stressors (starvation, bacteria injection, and heat shock) to determine the impact of restricted feeding on health and fitness.
The findings revealed that restricted feeding reduces the survival of flies in stressful conditions. In other words, restricted feeding increases the sensitivity of flies to stressors, despite having beneficial metabolic effects.
The functional impact of the microbiome was determined using the circadian phase shift paradigm (altered course of the day-night cycle). For the experiments, flies with and without microbiomes were used.
The findings revealed that the microbiome's presence increases circadian clock gene cycling in flies' brains. Overall, it was observed that sterile flies reset more rapidly with shifts in the day-night cycle compared to flies with a microbiome.
In other words, the gut microbiome modulates the gut clock response to alterations in the day-night cycle, facilitating the synchronization between gut and brain circadian rhythms.
Study significance
The study reveals that the gut microbiome in flies does not cycle. However, it regulates circadian rhythms of gene expression in the gut and prevents rapid fluctuations in response to different environmental conditions.
As the scientists in the journal mentioned, "these findings have important implications for common situations in the modern world."