
 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Background
The continual emergence of highly transmissible and immune-evasive Omicron subvariants, coupled with the waning of coronavirus disease 2019 (COVID-19) vaccine-elicited immunity, has increased BTI frequency among COVID-19 vaccinees. Previous studies on memory B lymphocyte- and antibody-mediated responses post-SARS-CoV-2 Delta or BA.1 BTIs have shown rapid anti-S humoral response recall, S-targeted memory B lymphocyte reactivation, and antibody-secreting cell differentiation.
Additionally, Omicron BTIs among fully vaccinated individuals enhance neutralization breadth, possibly due to novel antibodies targeting Omicron S epitopes or selective cross-protective memory B lymphocyte re-expansion during vaccination.
Evaluating the influence of BTIs with novel Omicron subvariants on the breadth and durability of humoral and memory B lymphocyte-mediated immunity could inform optimized vaccine development for maximum protection from emerging SARS-CoV-2 infections.
About the study
In the present study, researchers evaluate the initial recalled immune response kinetics after BA.1 or BA.2 BTIs and profiled alterations in SARS-CoV-2 neutralization post-recovery.
Twenty-six COVID-19 vaccinees, consisting of three fully vaccinated and 21 booster-vaccinated individuals and two individuals with prior SARS-CoV-2 ancestral strain infections who were subsequently vaccinated, were enrolled following Omicron BTIs occurring after a median of 95 days of receiving the most recent vaccination.
Among the study participants, 19 of 21 individuals had no history of SARS-CoV-2 infection and were vaccinated with S protein-encoding COVID-19 vaccines such as ChAdOx1 nCoV-19, BNT162b2, NVX-CoV2373, and messenger RNA (mRNA)-1273.
BA.1 infections were confirmed by whole genome sequencing (WGS). In addition, the nasal cavity and blood of the participants were sampled for at least 247 follow-up days.
SARS-CoV-2 ribonucleic acid (RNA) levels were determined using a quantitative polymerase chain reaction (qPCR) assay for the SARS-CoV-2 nucleocapsid (N) gene. Anti-Omicron neutralization was modeled to estimate the durability of immune protection against symptomatic reinfections with equivalently or more immune-evasive SARS-CoV-2 variants of concern (VOCs) among individuals with hybrid immunity.
Linear mixed effects modeling was performed to estimate the average decay rate. BTIs were diagnosed using rapid antigen tests and PCR, whereas anti-N titers were determined using enzyme-linked immunosorbent assays (ELISA).
The ancestral SARS-CoV-2 strain was cultured on Vero cells, whereas BA.1, BA.2, and BA.4 subvariants were grown on Calu3 cells. SARS-CoV-2 infectivity was assessed using HAT-24 cells expressing human angiotensin-converting enzyme 2 (ACE-2) and transmembrane serine protease 2 (TMPRSS2).
Percent viability and neutralization were determined based on the relative fluorescent units (RFU) values, and the mean ID50 values and IC50 values were determined. Flow cytometry was performed to detect SARS-CoV-2 S-targeted B lymphocytes, with modeled antibody recall and decay kinetics. Vaccine efficacy against different VOCs following BTI was estimated.
Results
All study participants developed symptomatic but mild BA.1 or BA.2 BTIs. These infections robustly enhanced their neutralization breadth against the causative VOCs, thereby expanding neutralization breadth against the more immune-evasive BA.4.
Cross-reactive memory B lymphocytes against the ancestral and Omicron S proteins were mainly expanded by SARS-CoV-2 infection, with limitedly recruited Omicron-targeted B lymphocytes or anti-SARS-CoV-2 antibodies de novo.
Modeling estimates indicated that immune protection against symptomatic reinfections with closely related VOCs would have considerable durability but would be undermined by novel and increasingly immune-evasive VOCs. Follow-up analyses indicated durable anti-Omicron neutralizing responses.
BA.1 and BA.2 BTIs showed comparable SARS-CoV-2 kinetics, with most infected individuals exhibiting peak viral loads at enrollment and up to three days following the onset of symptoms. SARS-CoV-2 replicated robustly during Omicron BTIs, despite previous vaccinations.
Serological anti-N immunoglobulin G (IgG) titers were negligible at the initial time points; however, the titers consistently expanded from one week after symptom onset of BA.1 and BA.2 BTIs. Immune protection was estimated to be over 70% and last for 705 days and 1,607 days after BA.1 and BA.2 BTIs, respectively.
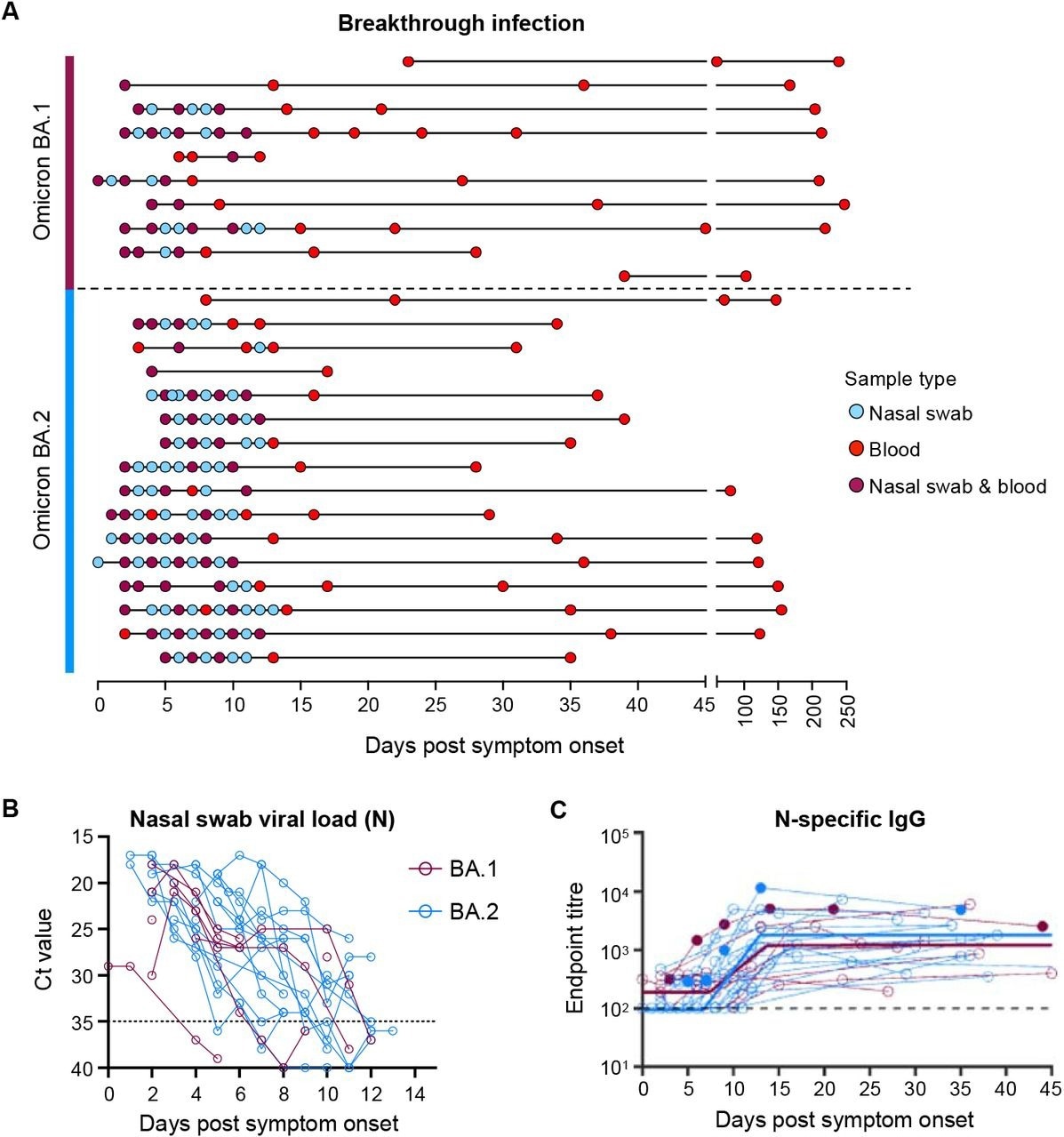
Viral load kinetics and seroconversion to N following Omicron BA.1 and BA.2 breakthrough infection. (A) Schematic of longitudinal sample collection following breakthrough infection of vaccinated individuals with Omicron BA.1 (n=10) or BA.2 (n=16). Each line represents a single subject, and each point represents a sample collection (blue, nasal swab; red, blood; purple, both nasal swab and blood). (B) Kinetics of SARS-CoV-2 viral load in nasal swabs measured by qPCR of the nucleocapsid (N) gene. (C) Kinetics of plasma IgG titers against SARS-CoV-2 nucleocapsid (N) following breakthrough infection with BA.1 (red) or BA.2 (blue). Subjects with previous SARS-CoV-2 infection are depicted in closed circles. The thick lines represent the mean estimate from the piecewise linear regression model using the estimated parameters.
Even during maximal SARS-CoV-2 neutralization, about one month following symptom onset, new VOCs with three- and 10-fold lower neutralization could lower the efficacy of immune protection from 90.0% to 74.0% and 47.0%, respectively. Among individuals with hybrid immunity from BA.1 and BA.2 BTIs, protective efficacy would reduce from 95.0% to 86.0% and 63.0%, respectively.
Recovery from Omicron BTIs conferred markedly durable immune protection from antigenically similar strains; however, the protection could be subverted rapidly by the emergence of more immune-evasive VOCs.
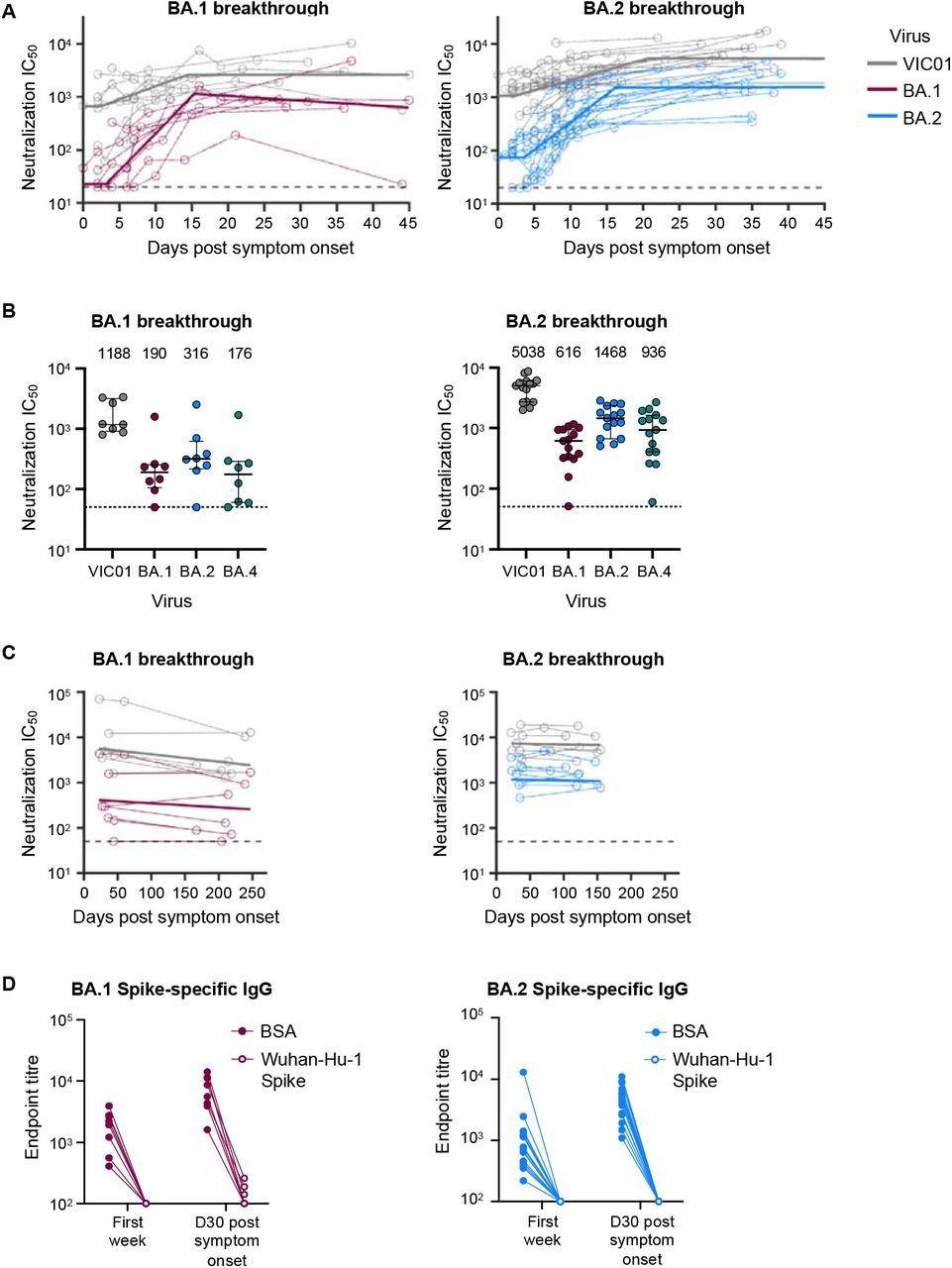
Omicron BA.1 and BA.2 breakthrough infection rapidly recalls neutralising antibodies that are broad and durable. (A) Kinetics of plasma neutralization activity following breakthrough infection against ancestral VIC01 or matched infecting Omicron BA.1 and BA.2 strains. Thick lines represent the mean estimate from the piecewise linear regression model using the estimated parameters. Plasma neutralization activity was measured using a live virus neutralization assay against SARS-CoV-2 clinical isolates in HEK293T cells transduced with ACE2 and TMPRSS2. (B) Neutralization mediated by BA.1 and BA.2 breakthrough plasma against ancestral VIC01, Omicron BA.1, BA.2, and BA.4 strains at a median of 34 days post-symptom onset. Data are presented as median ± IQR. (C) Longitudinal decay kinetics of plasma neutralization activity following breakthrough infection against ancestral VIC01 or matched infecting Omicron BA.1 or BA.2 strains up to 4-7 months post-symptom onset. The best-fit decay slopes (thick lines) are depicted. (D) IgG antibody endpoint titers against BA.1 spike for BA.1 breakthrough subjects (red) and against BA.2 spike for BA.2 breakthrough subjects (blue) following pre-incubation with BSA control (closed circles) or ancestral Wuhan-hu-1 spike (open circles).
The team estimated that 70% protective efficacy for the SARS-CoV-2 homologous strain could be maintained for nearly 4.5 and seven years for BA.1 and BA.2 BTIs, respectively. Likewise, durable immune protection for the SARS-CoV-2 ancestral strain was estimated to persist for over 8.5 years and 10 years for BA.1 and BA.2 BTIs, respectively.
Conclusions
Omicron BTIs were found to induce durable neutralizing immune responses by recalling cross-reactive and vaccine-induced memory B lymphocytes.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.