PD is associated with a gradual decline in motor control and several non-motor symptoms due to the progressive loss of dopaminergic neurons in the substantia nigra of the brain.
PD-related deaths have more than doubled since 2000, mainly because of the lack of good-quality interventions among the elderly. Thus, further research is needed to better understand the pathology of PD and develop early diagnostic systems.
The retina, often referred to as a window to the brain, provides a viable avenue for assessing neuropathological processes associated with many neurodegenerative diseases. Despite recent progress, clinical findings on retinal degeneration are not always inconclusive, which warrants further research to enhance retinal diagnostic power.
To this end, artificial intelligence (AI) algorithms, including deep learning models and conventional machine learning algorithms, have emerged as efficient diagnostic tools.
About the study
Developing a deep understanding of retinal biomarkers of PD requires a thorough knowledge of the structural degeneration of the retinal vasculature. Although this is often difficult to achieve clinically, AI could aid in elucidating the complex relationships at the local and global spatial levels of the retina. The present study proposes the use of AI algorithms to address the aforementioned challenge and is one of the first extensive AI studies on diagnosing PD from fundus imaging.
The study's primary aim was to systematically profile the classification performance across various phases of PD progression, including incident and prevalent PD. By neglecting any feature selection methods or external quantitative measures, the researchers maximized the diagnostic ability of AI algorithms. Robustness was established through deep learning and conventional machine learning methods.
Study findings
Deep neural networks outperformed conventional machine learning models and exhibited notable performance in the detection of PD in retinal fundus images. The model successfully predicted the incidence of PD before formal diagnosis with a sensitivity level of 80% from zero to 5.07 years.
Between 5.07 and 5.57 years, sensitivity rose to 93.33% and then reduced to 81.67% between 5.57 and 7.38 years. These results are promising, as they show the potential for early disease intervention.
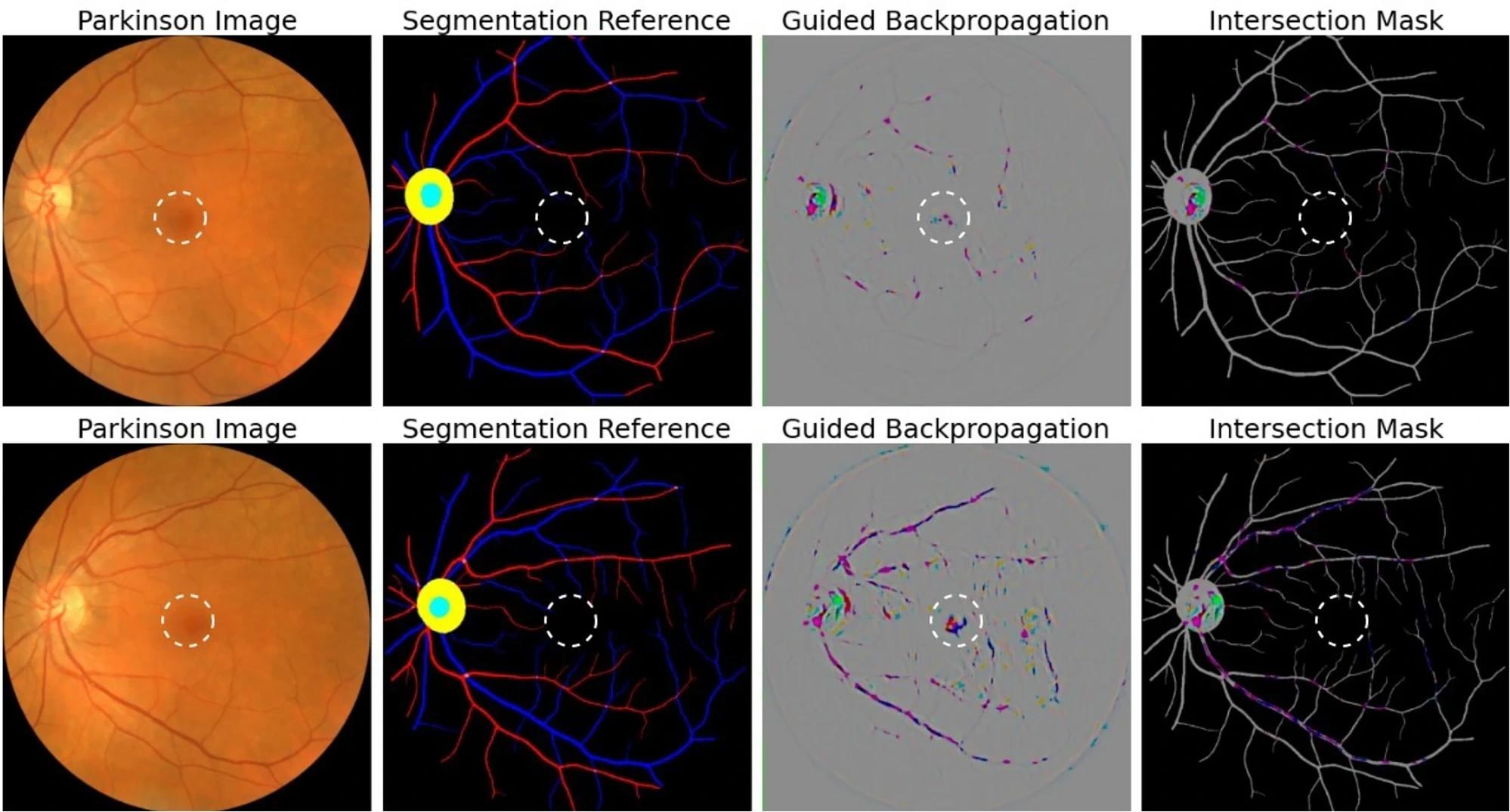 Attribution correspondence of retinal features. In the first column, an artery-vein (red and blue, respectively) map is combined with the optic cup (teal) and optic disc (yellow) generated from the AutoMorph deep learning segmentation module. A white dashed line is shown as an estimate for the foveal region. In the third column, a predicted attribution map is generated using the guided backpropagation algorithm on top of the AlexNet model. The intersection of the salient features with the segmentation is shown in the last column. The images represent the left (top) and right (bottom) eyes from the same subject, demonstrating distinct feature distributions for prediction.
Attribution correspondence of retinal features. In the first column, an artery-vein (red and blue, respectively) map is combined with the optic cup (teal) and optic disc (yellow) generated from the AutoMorph deep learning segmentation module. A white dashed line is shown as an estimate for the foveal region. In the third column, a predicted attribution map is generated using the guided backpropagation algorithm on top of the AlexNet model. The intersection of the salient features with the segmentation is shown in the last column. The images represent the left (top) and right (bottom) eyes from the same subject, demonstrating distinct feature distributions for prediction.
Automated deep neural networks can complement ophthalmologists to identify disease biomarkers and perform the high-throughput evaluation. To date, AI-based PD assessment using the retina is rare. Importantly, prior research did not compare deep learning and conventional machine learning methods.
In contrast, the current study evaluated a wide range of deep learning and conventional machine learning methods to consider the entire fundus image as a diagnostic medium. Moreover, prevalent and incident PD patients were successfully differentiated from appropriately matched healthy controls with an accuracy of 68%.
Conclusions
In the current study, conventional machine learning models were outperformed by deep learning models to precisely predict PD from retinal fundus images. This method was robust to image perturbations, which is promising for early treatment.
This work is expected to provide the foundation for future research and act as an algorithm selection reference for both its interpretability and performance.
A fundamental limitation of this study is the dataset size, which could be improved to capture a broader range of presentations of PD. Second, the study is based on the United Kingdom population, thereby limiting the generalizability of the findings.
An additional limitation of the current study is that the researchers did not report how this approach could be applied to different severity levels of PD. Although the current study was focused on PD, it remains unclear whether other neurogenerative diseases like Alzheimer's disease, as well as certain eye diseases, share similar degeneration patterns or biomarkers.
Future research should also investigate whether the model predictions can inform ophthalmologists' grading. However, this could be complicated as the visual biomarkers of common eye diseases are better understood than those of PD.
Taken together, these limitations necessitate additional research using diverse samples to establish the trustworthiness of AI models in clinical settings.
Journal reference:
- Tran, C., Shen, K., Liu, K., et al. (2024). Deep learning predicts prevalent and incident Parkinson’s disease from UK Biobank fundus imaging. Scientific Reports 14(1);1-12. doi:10.1038/s41598-024-54251-1