In a recent review published in the journal Advances in Nutrition, researchers examined the current evidence on the role of human milk oligosaccharides (HMOs) in protecting infants against respiratory syncytial virus (RSV) infection and disease, highlighting potential mechanisms and future research directions.
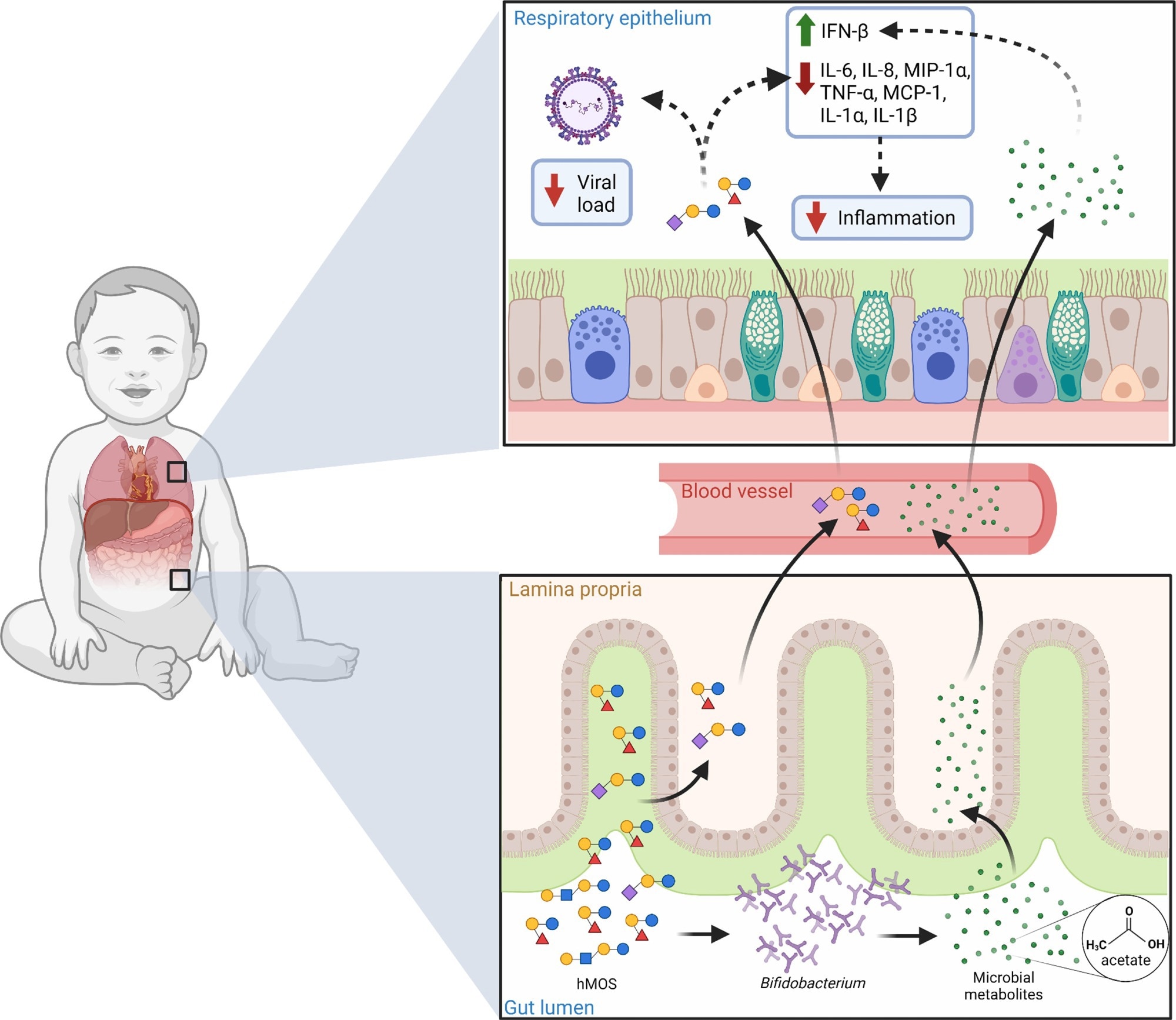 Potential role of hMOS on RSV disease. hMOS such as 2’-FL and LNnT are metabolized by Bifidobacterium in the infant’s gut into short-chain fatty acids, like acetate. Small quantities of hMOS and acetate are absorbed and can reach the lungs through the circulation, where they could act as antivirals and modulate inflammation. Small quantities of hMOS and short-chain fatty acids could also coat the upper respiratory mucosa in the form of regurgitated milk, as seen in the noses of breastfed infants. Review: Human milk oligosaccharides and respiratory syncytial virus infection in infants
Potential role of hMOS on RSV disease. hMOS such as 2’-FL and LNnT are metabolized by Bifidobacterium in the infant’s gut into short-chain fatty acids, like acetate. Small quantities of hMOS and acetate are absorbed and can reach the lungs through the circulation, where they could act as antivirals and modulate inflammation. Small quantities of hMOS and short-chain fatty acids could also coat the upper respiratory mucosa in the form of regurgitated milk, as seen in the noses of breastfed infants. Review: Human milk oligosaccharides and respiratory syncytial virus infection in infants
Background
RSV, a common cause of pediatric respiratory infections, particularly impacts infants under two years, with significant morbidity and mortality. Beyond the immediate health impact, RSV infection could also affect long-term immune development and overall health outcomes.
The heavy disease burden of RSV infection in infants, coupled with the lack of effective treatments, highlights the urgent need for prophylaxis strategies. Breastfeeding is shown to offer consistent protection against severe RSV disease, potentially owing to the bioactive components in breast milk, including HMOs. Recent studies have associated HMOs with lower respiratory infection risk and reduced viral load and inflammation in infants, highlighting their potential role in preventing and managing RSV infection.
Overview of human milk oligosaccharides
HMOs, abundant in human milk, play diverse roles in infant development. They are synthesized from lactose and can form various structures with additional sugars like GlcNAc, Gal, Fuc, and Neu5Ac. The concentration and composition of HMOs vary among individuals and populations due to genetic and environmental factors. HMOs are resistant to digestion and reach the colon intact, where they modulate the microbiome, inhibit pathogen binding, reduce inflammation, and modulate the immune system, potentially contributing to the prevention of viral infections in breastfed infants.
HMOs reduce the risk of respiratory infections
Clinical studies have explored the association between HMOs and respiratory symptoms in infants, particularly focusing on their potential preventive effects against RSV infection and other respiratory diseases. Lower levels of lacto-N-fucopentaose II (LNFP-II) in maternal milk and infant feces were found to be associated with increased respiratory symptoms in infants. Another study demonstrated that infant formula containing 2’-fucosyllactose (2’-FL) and lacto-N-neotetraose (LNnT) reduced the incidence of lower respiratory tract infections and bronchitis in infants. Additionally, the maternal secretor genotype, which affects the production of α1-2 fucosylated HMOs, was found to be associated with a reduced risk of acute respiratory infections in breastfed infants. However, some studies did not find a significant association between HMOs and respiratory infections. Further research is needed to elucidate the precise mechanisms and effects of HMO consumption on RSV incidence and severity, considering factors such as HMO composition, secretor status, and microbiome composition.
HMOs show antiviral activity
HMOs exhibit antiviral properties by binding to clinically relevant viruses, including rotavirus, norovirus, human immunodeficiency virus (HIV), and influenza. For instance, α1-2 fucosylated HMOs like 2’-FL can occupy norovirus binding sites, reducing infectivity. Additionally, certain HMOs compete with HIV-1 for binding sites on dendritic cells, potentially reducing transmission. Despite human milk's ability to transmit viruses, it rarely causes disease in infants, likely owing to the antiviral properties of HMOs. The structural diversity of HMOs provides a wide range of protection against viral infections, with implications for preventing diseases like coronavirus disease 2019 (COVID-19). However, research on HMOs's ability to preclude RSV infection and pro-inflammatory responses remains limited compared to other viruses.
Altering the host's innate response
Exposure to certain HMOs alters the response of human respiratory and peripheral blood mononuclear cells (PBMCs) to RSV infection. These HMOs are shown to reduce RSV viral load and cytokines linked to disease severity and inflammation in respiratory cells and PBMCs. Infants fed formula containing 2’-FL also exhibit lower plasma levels of inflammatory cytokines when challenged with RSV, similar to breastfed infants. Thus, HMO supplementation may enhance resistance to RSV infection in infants, potentially explaining the reduced risk of severe RSV disease observed in breastfed infants.
Modulation of gut microbiome to mitigate RSV disease severity
The gut-lung axis concept suggests that gut microbiota can influence immune defense against respiratory infections like RSV beyond the gastrointestinal tract. Changes in the gut microbiome and the associated metabolites are found to be linked to the incidence and severity of respiratory infections such as RSV.
Research on infant formula indicates that 2’-FL and LNnT can promote a Bifidobacterium-dominated microbiota in some infants, potentially reducing the need for antibiotics. Additionally, elevated fecal fucosylated glycans, lactate, acetate, and Bifidobacterium are associated with reduced risk of bronchitis or lower respiratory tract infections in infants.
Acetate, produced by gut bacteria in response to specific HMOs, may enhance immune responses against RSV infection. Animal studies demonstrate that acetate supplementation can protect against RSV-induced lung inflammation, and clinical observations in infants with RSV bronchiolitis suggest that high levels of fecal acetate are associated with milder symptoms.
Conclusion and future perspectives
HMOs show promise in combating RSV through multiple mechanisms, including direct antiviral action and gut microbiota modulation. Standardized methods for identifying HMOs are essential. Future studies should optimize designs to investigate HMOs effects on RSV. Extensive birth-cohort studies could provide valuable insights. Key questions include identifying specific HMOs protective against RSV and understanding their mechanisms of action.