On April 29th, 2024, the U.S. FDA granted full approval for Seagen Inc.'s TIVDAK® (tisotumab vedotin) targeting tissue factor (TF) for the treatment of patients with recurrent or metastatic cervical cancer who have progressed on or after chemotherapy. This marks a significant advancement in the therapeutic landscape for cervical cancer, highlighting the potential of antibody-drug conjugates (ADCs) in oncology.
TIVDAK’s mechanism of action
Tivdak is an ADC drug that targets TF, combining Genmab's TF-targeting monoclonal antibody tisotumab with Seagen's ADC technology, which is designed to target TF antigens on cancer cells and deliver the cytotoxic payload MMAE directly into cancer cells.
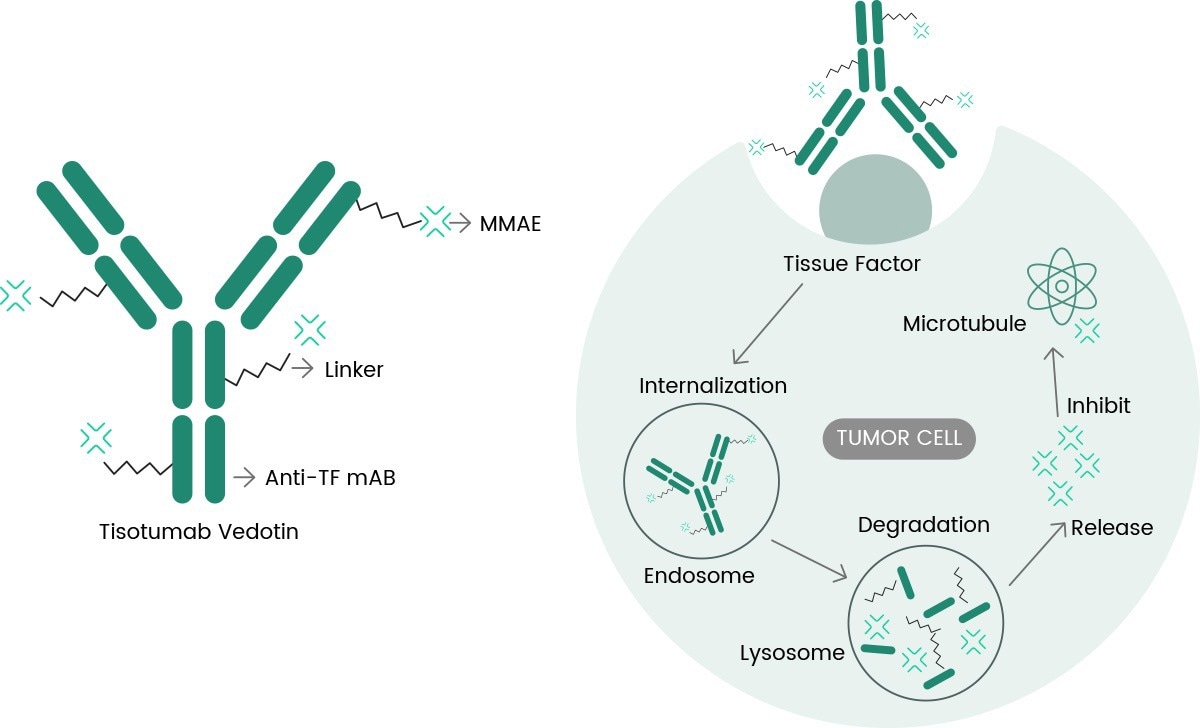 Tisotumab Vedotin molecular pathway of action (https://doi.org/10.3390/ijms23073559)
Tisotumab Vedotin molecular pathway of action (https://doi.org/10.3390/ijms23073559)
TF: ideal target for ADC development
TF is known to be involved in tumor signaling and angiogenesis pathways and overexpressed in the vast majority of patients with cervical cancer and in many other solid tumors. Its ability to internalize rapidly when bound by antibodies and the minimal impact on normal coagulation processes further enhance its suitability for targeted cancer therapies.
Sino Biological’s contribution to tissue factor drug development
Sino Biological is at the forefront of advancing drug development targeting tissue factor, providing an extensive array of recombinant TF proteins and a diverse range of TF antibodies, to support the scientific community in pioneering new therapeutic strategies. Partner with Sino Biological and visit our website for further information about high-quality TF proteins and antibodies to accelerate your drug development.