In August 2024, the WHO declared Mpox (formerly known as Monkeypox) a global health emergency due to rising Clade I Mpox cases in Africa and Sweden, highlighting the urgency for coordinated global efforts to monitor and mitigate the spread of the virus.
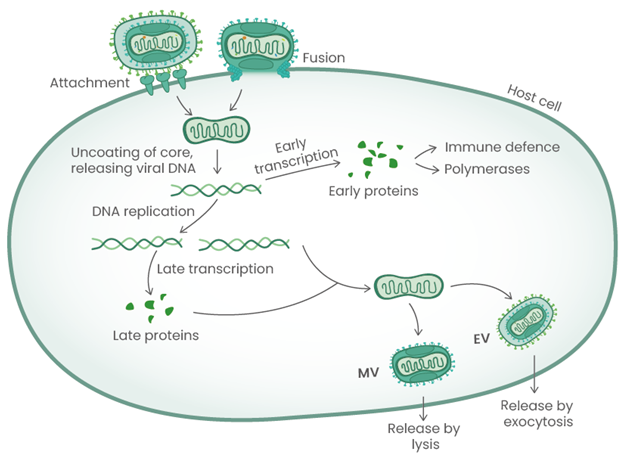 The MPXV life cycle with the different viral antigens playing role at the indicated steps. Image Credit: Sino Biological
The MPXV life cycle with the different viral antigens playing role at the indicated steps. Image Credit: Sino Biological
Current vaccines and the need for continued research
The Mpox virus belongs to the Orthopoxvirus genus, within the Poxviridae family, and shares a close relationship with the variola virus, which causes smallpox. The primary vaccine used to prevent Mpox is JYNNEOS, which is a two-dose vaccine developed to protect against both Mpox and smallpox. However, the emergence of more virulent strains underscores the need for ongoing research to ensure that existing vaccines remain effective.
Continued research is crucial not only for vaccine efficacy but also for understanding the evolving pathogenicity and transmission dynamics of the Mpox virus. The virus's ability to spread in previously low-risk populations, including children, and its increasing virulence demand the development of new therapeutic strategies and the refinement of existing vaccines.
Understanding Mpox virus
Mpox virus is an enveloped, double-stranded DNA virus existing in two forms: the intracellular mature virion (MV) and the extracellular enveloped virion (EV). MPXV enters host cells through attachment and fusion, with MVs typically released upon cell lysis and EVs exiting cells via exocytosis. Key viral proteins, homologous to those of the vaccinia virus (VACV), such as A35R, A29, B6R, M1R, H3L, and L1R, play critical roles in the virus's lifecycle. These proteins are involved in promoting virulence, facilitating cell entry, supporting viral replication, and evading the host immune response, making them important targets for research and drug development.
Currently, MPXV is diagnosed using PCR, the gold standard, alongside immunodetection methods that offer quicker, more accessible, and cost-effective alternatives by detecting viral antigens directly in biological samples. The development of more sensitive and specific viral proteins and antibodies is crucial for improving diagnostic techniques and advancing vaccine development, supporting the creation of affordable, easily manufactured vaccines for global distribution.
Therapeutic targets for Mpox
Identifying and understanding key therapeutic targets within the Mpox virus is essential for developing effective treatments and vaccines. These targets include:
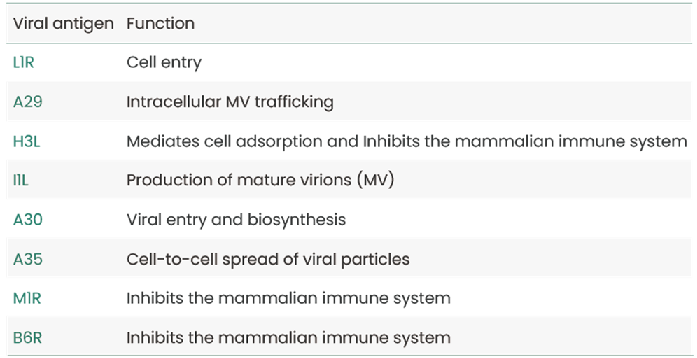
- Viral entry proteins: such as L1R and A35R are crucial for the virus’s entry into host cells. Inhibiting these proteins could prevent infection at its earliest stage.
- Proteins involved in viral replication: I1L and A29 are crucial for viral replication and trafficking within the host cell. Disrupting these proteins could reduce viral load and disease severity.
- Immune evasion mechanisms: Mpox uses proteins like M1R and B6R to evade the host's immune system. Neutralizing these proteins could enhance the body’s ability to clear the infection.
- Vaccine development: The same viral proteins can serve as epitopes in vaccine development, training the immune system to recognize and attack the virus, providing immunity against Mpox.
- Advances in antigenic targeting: Recent research using high-throughput screening methods is aimed to identify novel antigens within the Mpox virus, crucial for discovering new drug targets and developing next-generation vaccines.
Table 1. Known Mpox Virus Antigens and Their Roles in Viral Infection. Source: Sino Biological
Sino Biological’s role in Mpox research
Sino Biological is at the forefront of accelerating Mpox research, offering a comprehensive portfolio of MPXV proteins and antibodies. This includes various recombinant MPXV proteins with different tags to meet diverse research needs. Additionally, Sino Biological has developed over 30 antibodies targeting various MPXV antigens, validated for applications such as LFA, ELISA, and WB assays.
Sino Biological’s Featured MPXV Products:
- mAb pairs tested on Lateral Flow Assay (LFA)
The following mAb pairs targeting MPXV A29 have been tested on Lateral Flow Assay (LFA) with a detection sensitivity of 10-100 pg/mL.
Source: Sino Biological
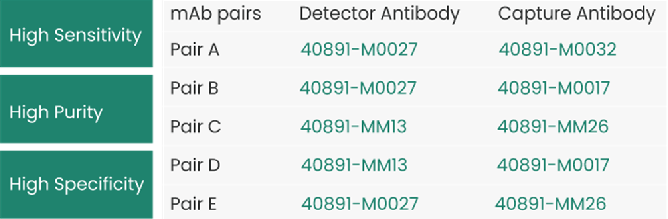
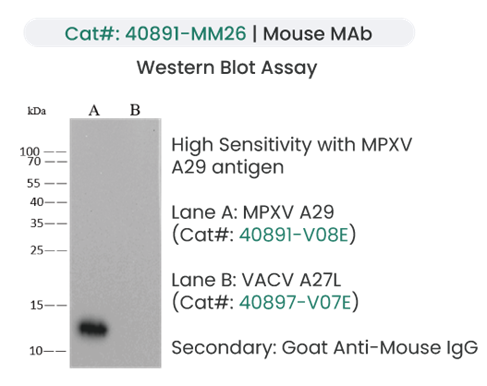
Image Credit: Sino Biological
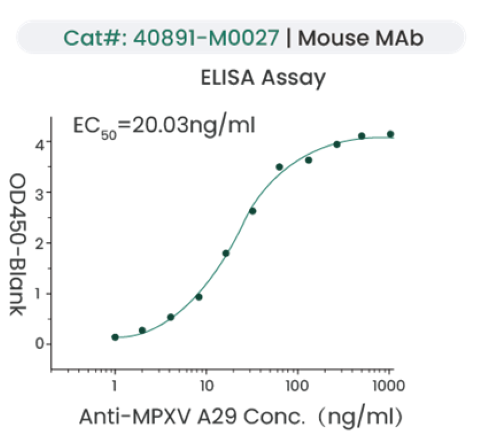
Image Credit: Sino Biological
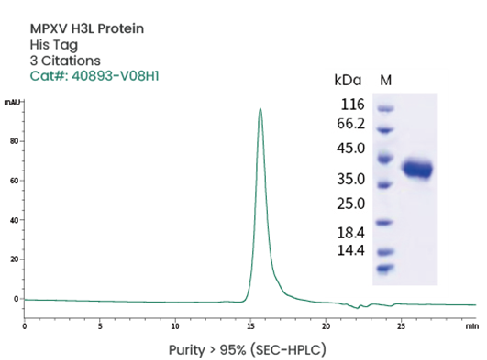
Image Credit: Sino Biological
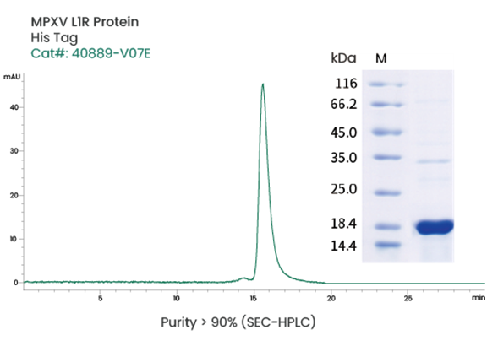
Image Credit: Sino Biological