On July 9th, 2024, the U.S. FDA approved Arcutis Biotherapeutics' ZORYVE® (roflumilast) for treating atopic dermatitis (AD), the most common type of eczema affecting millions in the U.S. This once-daily, steroid-free cream could become a new standard of care for AD. The approval underscores the growing importance of researching Phosphodiesterase 4 (PDE4) and other PDEs as immunomodulatory targets for various inflammatory diseases. Sino Biological, and its subsidiary SignalChem Biotech, support drug discovery and development targeting PDEs by providing a broad range of PDE families and isoforms.
Mechanism of action
Roflumilast is a phosphodiesterase-4 (PDE4) inhibitor that reduces inflammation by increasing cyclic adenosine monophosphate (cAMP) levels, an important molecule in regulating inflammation. PDE4, which breaks down cAMP, has four subtypes (PDE4A, PDE4B, PDE4C, PDE4D) and about 20 isoforms. The anti-inflammatory effects of PDE4 inhibitors are mainly due to inhibiting PDE4B and PDE4D, which are highly expressed in immune cells. By inhibiting these subtypes, roflumilast raises cAMP levels, decreasing pro-inflammatory mediators (e.g., TNF alpha, IL-4, IL-5, IL-13, IL-17A, IL-17F, IL-22) and increasing the anti-inflammatory mediator IL-10. This helps manage inflammatory conditions like atopic dermatitis, alleviating symptoms such as redness, itching, and swelling.
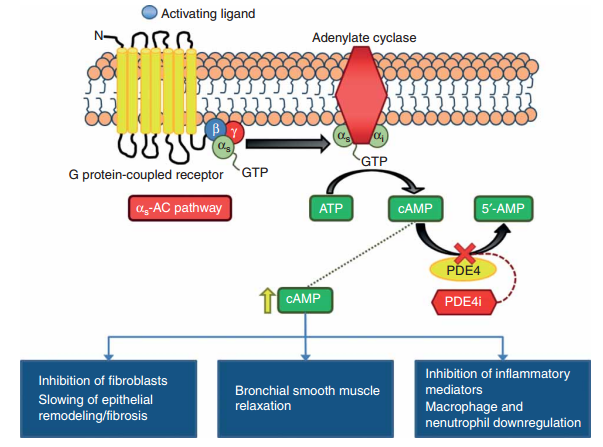 PDE4 inhibitors mechanism of action. Image Credit: DOI:10.1517/13543784.2015.1094054
PDE4 inhibitors mechanism of action. Image Credit: DOI:10.1517/13543784.2015.1094054
Phosphodiesterases (PDEs) as drug targets
PDE enzymes control essential body functions by managing cAMP and cGMP levels. Various PDE inhibitors are developed for diseases: PDE1/2 for cardiovascular and neurological disorders; PDE4 for COPD and atopic dermatitis; PDE5 for erectile dysfunction and pulmonary arterial hypertension; PDE6 for retinal diseases; and PDE7 for inflammatory and autoimmune problems. In summary, PDE inhibitors are powerful tools and hold great promise as drug targets for wide-ranging therapeutic applications.
Sino Biological’s offering to support Phosphodiesterases (PDEs) research
Sino Biological, along with its subsidiary SignalChem Biotech, is at the forefront of advancing drug development, offering a wide variety of recombinant PDE families and isoforms, to facilitate research on PDEs. Partner with us and visit our website to learn more about how we can best work together to accelerate the PDEs research.