Gilead Sciences announced a significant milestone: the U.S. FDA accepted their New Drug Applications (NDAs) for lenacapavir (LEN), a groundbreaking twice-yearly injectable HIV-1 capsid inhibitor for pre-exposure prophylaxis (PrEP). The FDA granted priority review, with a decision expected by June 19, 2025. LEN represents a novel class of HIV therapies by targeting the viral capsid, a multifunctional protein essential for HIV replication. Unlike traditional antiretrovirals that target enzymes such as reverse transcriptase or protease, LEN binds directly to the capsid protein, disrupting several critical steps in the virus’s life cycle.
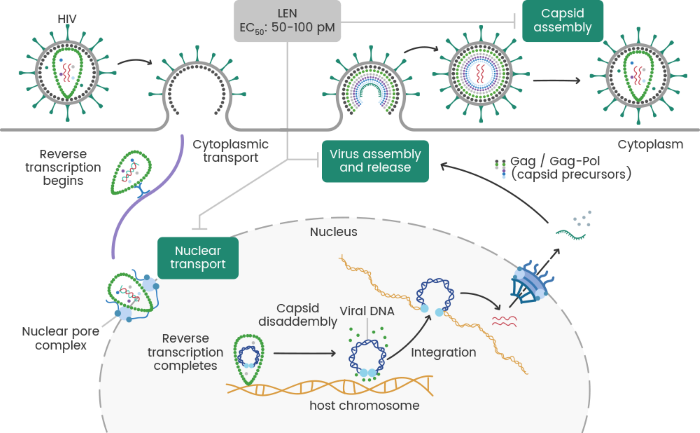 Figure 1. Mechanism of Action of LEN. Image Credit: Sino Biological Inc.
Figure 1. Mechanism of Action of LEN. Image Credit: Sino Biological Inc.
At Sino Biological, we are proud to support this era of progress with our industry-leading recombinant HIV antigen[ products. As the fight against HIV evolves, our high-quality antigens empower researchers, vaccine developers, and diagnostic innovators to stay ahead of the curve.
Mechanism of action
LEN is a first-in-class, long-acting HIV-1 capsid inhibitor that disrupts multiple stages of the viral replication cycle. By binding to the capsid protein, it blocks the nuclear import of HIV-1 proviral DNA, preventing its integration into the host genome. Additionally, it disrupts capsid core formation, resulting in structurally defective virions, and interferes with Gag/Gag-Pol function, reducing proper virus assembly and release. This multistage inhibition gives LEN potent antiviral activity, even against drug-resistant HIV-1 strains. Administered subcutaneously every six months after an oral lead-in phase, its extended half-life (8–12 weeks) ensures sustained viral suppression, offering a promising option for individuals with multidrug-resistant HIV or those seeking alternatives to daily therapy (Figure 1).
Why choose Sino Biological HIV antigens?
- Premium Quality: Our recombinant HIV antigens, including envelope glycoproteins, reverse transcriptase enzymes, and capsid proteins, are produced with rigorous purity and functionality standards, ensuring reliable results.
- Versatile Applications: Ideal for vaccine research, assay development, and drug screening studies.
- Trusted Partner: With years of expertise, Sino Biological delivers consistent, scalable products to accelerate your HIV projects.
Featured product: HIV-1 envelope glycoprotein
- Highly purified, biologically active
- Ideal for drug screening, assay development, and vaccine research
- Available in bulk for large-scale projects
Research application
Sino Biological products are frequently cited in well-reputed journals. For instance, Yang, C., Liu, D., and colleagues used recombinant HIV capsid protein p24 (Sino Biological ) and an HIV-1 p24 ELISA Pair Set to develop a gold nanorod (AuNR)-based microfluidic-integrated multicolor immunosensor for HIV-1 p24 detection with patients’ serum samples. Their study demonstrated that increased levels of HIV-1 p24 are detectable within 1-2 weeks after infection (Figure 2) 1.
In another study, McKendry, R., and collaborators developed novel nanobodies to detect HIV-1 p24. They assessed these nanobodies' ability to detect multiple p24 subtypes using an indirect enzyme-linked immunosorbent assay (ELISA) with purified recombinant HIV-1 p24 proteins, including Group M subtype D, as well as Groups N and O (Sino Biological) (Figure 3) 2.
Additionally, Li, L., and coworkers discovered that a glycosylated dihydrochalcone, trilobatin, inhibits HIV-1 entry by blocking the gp41 pocket-forming site. They further evaluated the possible mechanisms of action in gp120-mediated HIV-1 entry and found that trilobatin did not bind to the gp120 envelope. In contrast, the positive control, HP-OVA, bound to HIV gp120 (Sino Biological) in an ELISA assay (Figure 4)3.
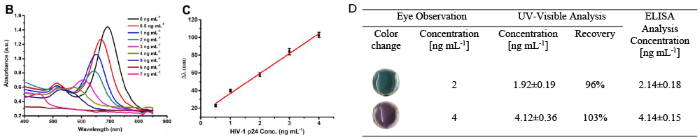
Figure 2. (B) UV-visible spectra of etched AuNRs for detecting 0-16 ng/mL HIV-1 p24. (C) Linear relationship between ∆λ and HIV-1 p24 concentration (R2=0.967). (D) Analytical results of HIV-1 p24 in serum samples. Image Credit: DOI: 10.1021/acs.analchem.0c02091
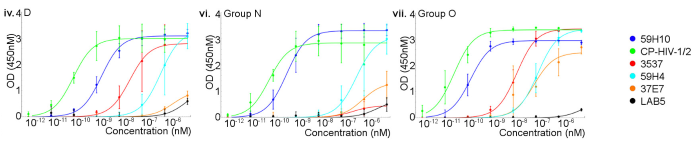
Figure 3. Comparison of binding of different p24 subtypes by four nanobodies and two p24-binding mAbs. Results from an indirect ELISA for Group M subtype D, Group N, and O (NDK, 06CM-U14296, BCF06; Sino Biological) with nanobodies 59H10, 37E7, 59H4, LAB5, and mAbs NIH-3537, Capricorn HIV-1/2 (CP-HIV-1/2). Image Credit: DOI: 10.1021/acsinfecdis.6b00189
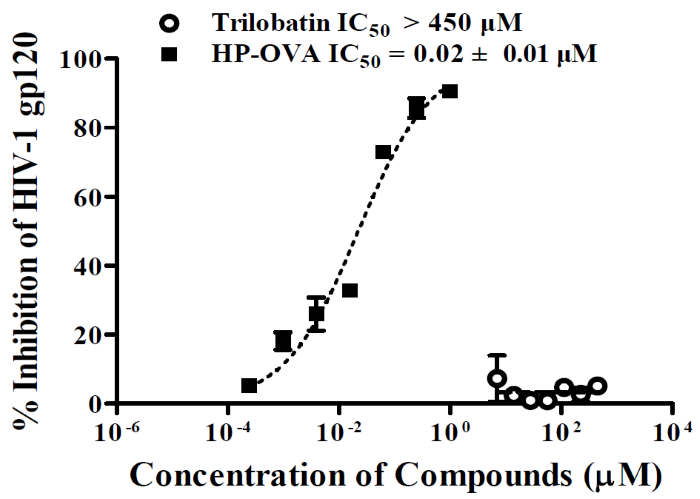
Figure 4. Inhibitory activities of trilobatin against HIV-1 gp120 (Sino Biological). HP-OVA served as a positive control. Image Credit: DOI: 10.1002/1873-3468.13113
Sino Biological is dedicated to fostering collaborations that drive impactful breakthroughs in HIV and other viral research. Leveraging our high-quality reagents and cutting-edge technologies, we are committed to supporting your scientific endeavors.
References
- Liu, D., et al. (2020). Microfluidic-Integrated Multicolor Immunosensor for Visual Detection of HIV-1 p24 Antigen with the Naked Eye. Analytical chemistry, [online] 92(17), pp.11826–11833. https://doi.org/10.1021/acs.analchem.0c02091.
- Gray, E.R., et al. (2017). Unravelling the Molecular Basis of High Affinity Nanobodies against HIV p24: In Vitro Functional, Structural, and in Silico Insights. ACS infectious diseases, [online] 3(7), pp.479–491. https://doi.org/10.1021/acsinfecdis.6b00189.
- Yin, S., et al. (2018). Trilobatin as an HIV-1 entry inhibitor targeting the HIV-1 Gp41 envelope. FEBS Letters, 592(13), pp.2361–2377. https://doi.org/10.1002/1873-3468.13113.