Study findings elucidated that the rapid eye movement (REM) sleep stage was closely associated with connections within and between the default mode network (DMN), the cingulo-opercular network (CON), and visual and auditory networks. They further revealed that the thalamus played a central role in the REM connectome and acted as a relay station for sensory information during REM. While not the first to establish a relationship between REM sleep loss and DMN connectivity, this study identified late-night sleep loss as having the most profound effect on the latter, which in turn may exacerbate the risk and intensity of psychiatric disorders.
 Study: The impact of REM sleep loss on human brain connectivity. Image Credit: Stokkete / Shutterstock
Study: The impact of REM sleep loss on human brain connectivity. Image Credit: Stokkete / Shutterstock
Background
Sleep deprivation is a hidden pandemic in today's fast-paced world, with research highlighting that more than 30% of adults fail to achieve adequate sleep. The condition represents a substantial public health concern due to the profound effects sleep deprivation is observed to have on individuals' physical and mental well-being. Sleep loss as an outcome of psychosocial stress, shifts in work timings, and, most notably, excessive electronic media consumption have been hitherto linked with obesity, heightened risk of metabolic diseases, and disruptions in emotional processes.
Unfortunately, a substantial portion of these outcomes are derived from anecdotal or observational evidence, with limited systematic research on the impacts of sleep disruptions on dynamic reorganizations of key brain components. Recent studies have aimed to elucidate how the two distinct sleep phases – rapid eye movement (REM) and non-REM (NREM; also called slow-wave sleep [SWS]) are linked to the duration and time of sleep and have suggested that the latter predominates early-night periods, while the former occurs later during the night. While science has elucidated the importance of REM sleep in maintaining the brain's energy balance and clearing active-state metabolic byproducts, the association between REM and brain function remains poorly understood.
About the study
In the present study, researchers used the split-night paradigm. This study protocol separates rapid eye movement (REM) and non-rapid eye movement (NREM) sleep to answer two main questions:
- What are the specific brain regions associated with REM?
- How do REM sleep disruptions (particularly during the late-night period) impact REM-associated brain networks compared to adequate sleep?
The study cohort was derived from right-handed adult volunteers recruited from six Beijing-based universities. After the screening, 113 volunteers were included in the study and randomly assigned to one of three investigation cohorts – the late-night sleep deprivation group (n = 41; sleep duration from 23:00 to 03:30), full-night sleep (n = 36; 23:00 to 08:00), and early night sleep deprivation cohort (n = 36; 03:00 to 07:30). Participants were required to refrain from alcohol, drug, and caffeine consumption for two days before study initiation. All participants were subjected to experimental investigations at 08:00 the morning following the sleep restriction intervention. Participants' regular sleep pattern data was recorded using sleep actigraphy and a seven-day-long sleep diary.
Experimental investigations comprised Resting-State Functional MRI (rs-fMRI) scans to identify regional brain interactions following sleep restriction interventions. Somte polysomnographic (PSG) mobile recording systems were used to measure and record electroencephalography (EEG) readings (F3, F4, C3, C4, O1, O2), electrooculography (EOG), and electromyography (EMG) data. The American Academy of Sleep Medicine (AASM) guidelines were followed when measuring and manually scoring participants' sleep stages.
The above-obtained data was used to partition participants' brains into 227 regions comprising ten brain networks. These networks included the default mode network (DMN), the dorsal- and ventral attention networks (DAN and VAN), the visual network (VIS), and the auditory network (AUD). Neural network connectivity patterns were elucidated using the Connectome-based Predictive Modeling (CPM) of rs-fMRI data.
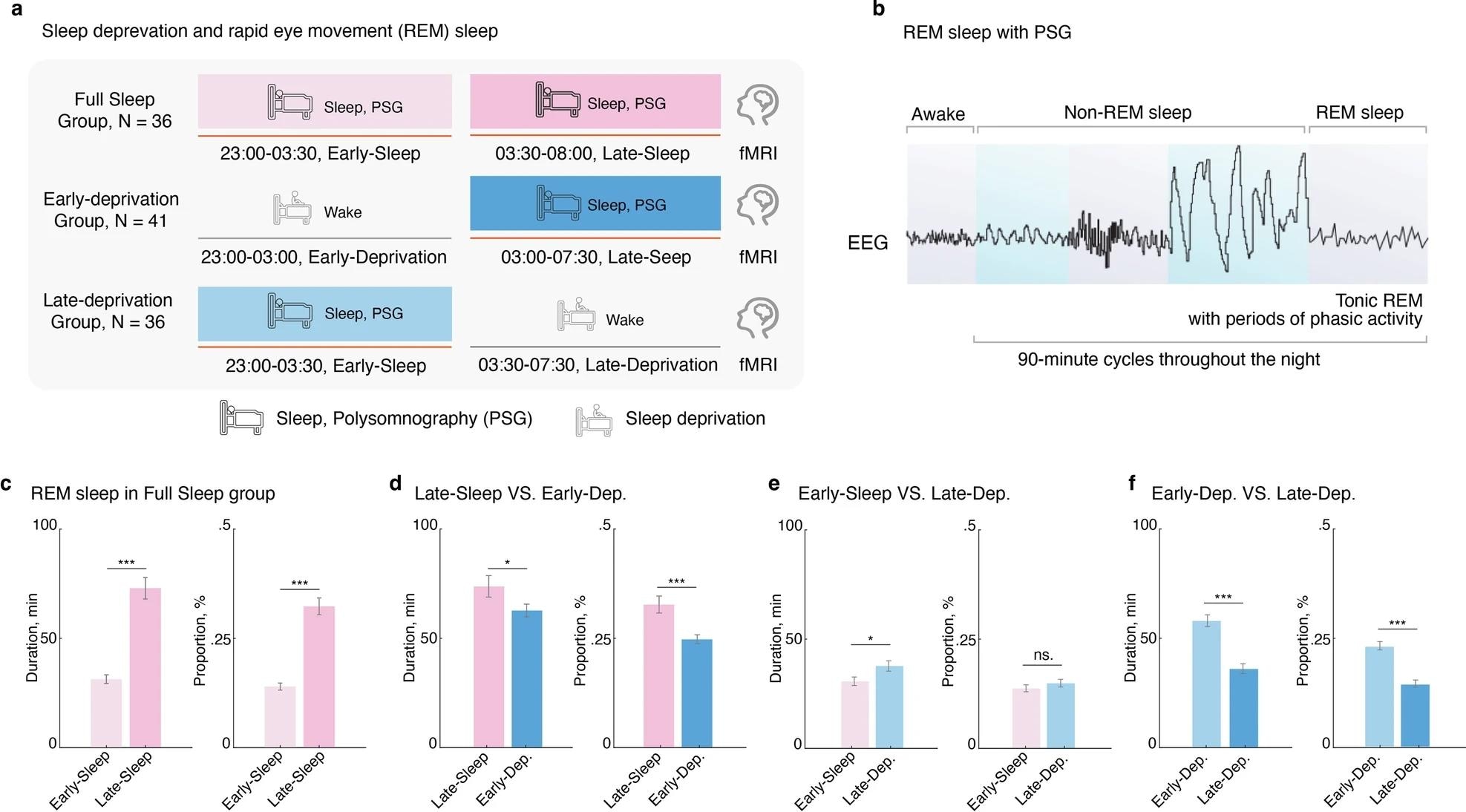 Late-night REM sleep, but not early-night REM sleep, maintains optimal REM sleep patterns.. a Experimental timeline for manipulating REM sleep loss procedure. b Diagram for the sleep progression from awake to non-rapid eye movement (NREM), and rapid eye movement (REM) sleep, along with the changes in electroencephalography (EEG), was tracked using Polysomnography (PSG) over a 90-minute cycle. c Comparison of REM phase duration and percentage between early-night and late-night sleep in the Full-Sleep Group, revealing higher values during early-night sleep. d Decreased REM phase duration and percentage in the Early-Deprivation Group compared to late-night sleep in the Full-Sleep Group. e Increased REM phase duration and percentage in the Late-Deprivation Group compared to early-night sleep in the Full-Sleep Group. f Significantly better REM phase duration and percentage in the Early-Deprivation Group compared to the Late-Deprivation Group. *p < 0.05; ***p < 0.001. ns. Not Significant. Data are presented as the mean ± SEM.
Late-night REM sleep, but not early-night REM sleep, maintains optimal REM sleep patterns.. a Experimental timeline for manipulating REM sleep loss procedure. b Diagram for the sleep progression from awake to non-rapid eye movement (NREM), and rapid eye movement (REM) sleep, along with the changes in electroencephalography (EEG), was tracked using Polysomnography (PSG) over a 90-minute cycle. c Comparison of REM phase duration and percentage between early-night and late-night sleep in the Full-Sleep Group, revealing higher values during early-night sleep. d Decreased REM phase duration and percentage in the Early-Deprivation Group compared to late-night sleep in the Full-Sleep Group. e Increased REM phase duration and percentage in the Late-Deprivation Group compared to early-night sleep in the Full-Sleep Group. f Significantly better REM phase duration and percentage in the Early-Deprivation Group compared to the Late-Deprivation Group. *p < 0.05; ***p < 0.001. ns. Not Significant. Data are presented as the mean ± SEM.
Study findings
REM sleep pattern segment analysis revealed that the duration and proportion of REM sleep were significantly higher in late-night sleep compared with early-night sleep. When partitioning the full-night sleep (FS) group data into early-FS and late-FS and comparing late-FS data with early- and late-night sleep cohorts, findings revealed that the early-deprivation group depicted significant decreases in both duration and proportion of REM sleep state, while the late-deprivation group only depicted reductions in duration.
However, early deprivation patterns were significantly better overall for REM outcomes than late deprivation. Together, these findings highlight that while both early- and late deprivation negatively impact REM sleep states, the early deprivation pattern is preferred when lifestyles or occupations necessitate sleep deprivation. Multi-level characterization of the REM sleep connectome revealed that the CPM predominantly resides in the DMN-DMN and CON-CON networks but is also found in subcortical (SUB)-VIS networks. Surprisingly, the thalamus and visual/auditory cortex were revealed to play a vital role in CPM predictions and, in turn, the REM connectome.
"…we observed that the thalamus exhibited the highest degree centrality and made a significant contribution to the REM connectome. Additionally, the subcortical networks, to which the thalamus belongs, displayed the third most prominent predictive edges. During REM sleep, the thalamus acts as a relay station for sensory information, transmitting signals from the environment to the cerebral cortex. It is involved in regulating the transition between different sleep stages, including the onset and termination of REM sleep cycles."
The study does have a notable limitation in that it only measures brain activity and connectivity without investigating behavioral changes (e.g., cognition or memory). This limitation notwithstanding, the study provides the groundwork for future assessments of both psychiatric and NREM evaluations.
Conclusions
The present study highlights the impacts of early and late sleep deprivation on REM sleep patterns by elucidating the network connectivity and impacted brain regions during these increasingly common suboptimal behaviors. Study findings reveal that sleep deprivations and disruptions adversely impact the DMN network and may negatively alter thalamus function. In summary, this study widens our understanding of how REM sleep phases maintain or modify variabilities in normal brain functioning.