In a recent study published in the journal PLOS ONE, researchers from the United States of America investigated the antiviral effects of valproic acid (VPA), alone and in combination with docosahexaenoic acid (DHA), against infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). They found that VPA, especially when combined with DHA, reduced SARS-CoV-2 infection rates and severity and potentially activated intracellular antiviral mechanisms by modifying gene expression.
Background
Initially used as an inert excipient, VPA was discovered in the 1960s to have antiseizure properties. It is now used for treating seizure disorders, bipolar disorder, and migraines. It has a known toxicity profile, including a three-fold increased risk of congenital disabilities, likely due to its histone deacetylase (HDAC) inhibition, making it contraindicated in pregnant women. VPA's HDAC inhibitory activity is also being explored for cancer therapy and human immunodeficiency virus (HIV) treatment. Additionally, VPA has antiviral activity against various viruses, including DNA (short for deoxyribonucleic acid), RNA (short for ribonucleic acid), and enveloped viruses. Efforts to repurpose drugs approved by the Food and Drug Administration (FDA) for coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2, have identified VPA as a potential candidate due to its interaction with the virus's nsp5 protease. However, previous in vitro viral replication assays did not demonstrate antiviral activity for VPA at the doses tested.
In the present study, researchers conducted in vitro testing and epidemiologic analysis to investigate VPA's antiviral activity alone and in combination with DHA. They identified pitfalls in screening methods and proposed a potentially cost-effective strategy against certain coronaviruses.
About the study
In the present study, the cytotoxicity of VPA was assessed using the XTT (short for methoxynitrosulfophenyl-tetrazolium carboxanilide) assay. VPA's half maximal inhibitory concentration (IC50) was determined using antibody-based, luciferase, and dot blot assays on Vero and MRC-5 cells infected with SARS-CoV-2 or human coronavirus 229E (HCoV-229E). HDAC activity was measured using a fluorometric assay kit. The impact of polyunsaturated fatty acids (PUFAs) like DHA, eicosapentaenoic acid (EPA), linoleic acid (LA), and alpha-linolenic acid (αLA) on HCoV-229E replication was evaluated. RNA was extracted, sequenced, and analyzed for differential gene expression changes. Protein expression was confirmed by Western blotting. The effects of VPA and DHA on HCoV-229E replication were studied using RNA sequencing (RNAseq). MRC-5 cells were pre-treated with DHA, VPA, or both, then infected with SARS-CoV-2 to evaluate replication inhibition, with results analyzed by real-time reverse transcription polymerase chain reaction (RT-PCR) and RNAseq.
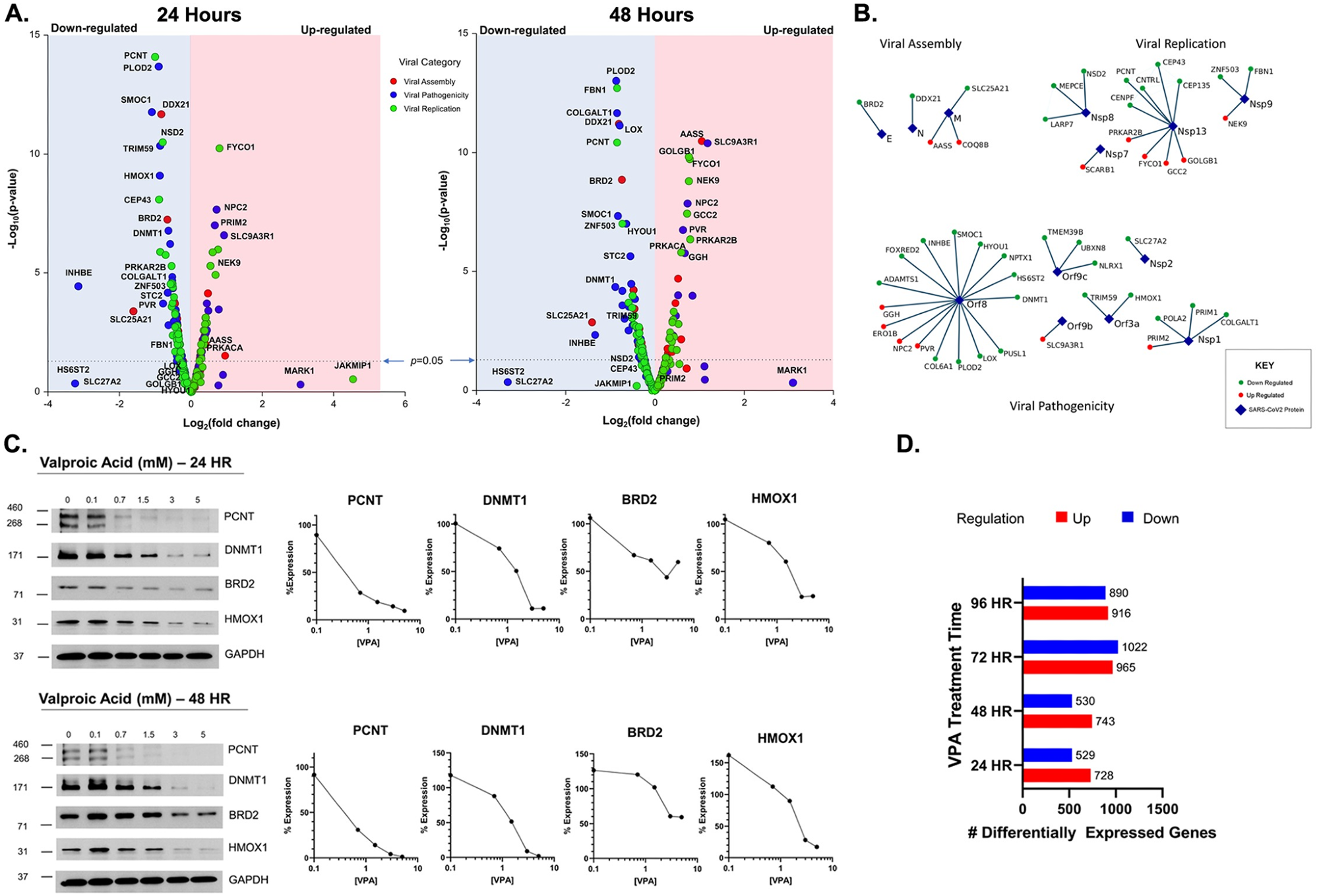 Host cellular genes interactions with the SARS-CoV2 proteins. (A.): Volcano plot for gene expression levels of selected 300 Gordon gene setat 24 hours (left) and 48 hours (right). (B.): Virus-host protein interaction map of the Gordon gene sets that met significance criteria for Viral Assembly, replication and pathogenicity. (C.) Protein levels measured by western blot for selected gene sets PCNT, DNMT1, BRD2, and HMOX1 at 24 hours (top) and 48 hours (bottom). (D.) Differentially expressed genes upon VPA treatment for 24, 48, 72 and 96 hours.
Host cellular genes interactions with the SARS-CoV2 proteins. (A.): Volcano plot for gene expression levels of selected 300 Gordon gene setat 24 hours (left) and 48 hours (right). (B.): Virus-host protein interaction map of the Gordon gene sets that met significance criteria for Viral Assembly, replication and pathogenicity. (C.) Protein levels measured by western blot for selected gene sets PCNT, DNMT1, BRD2, and HMOX1 at 24 hours (top) and 48 hours (bottom). (D.) Differentially expressed genes upon VPA treatment for 24, 48, 72 and 96 hours.
Further, epidemiologic data from the Optum dataset were analyzed to study COVID-19 outcomes associated with VPA use, and serum VPA levels were obtained from LabCorp for analysis.
Results and discussion
In vitro, VPA's IC50 for inhibiting HDAC2 was found to be 2.5 mM, higher than therapeutic levels. Although initial viral inhibition assays did not show effective viral replication inhibition at tested doses, preincubation with VPA was observed to reduce the IC50 by over three-fold, approaching therapeutic levels. VPA's impact on gene expression suggested potential antiviral activity, although direct inhibition of SARS-CoV-2 was not demonstrated at therapeutic doses.
Additionally, LA and DHA were found to significantly inhibit viral replication when tested on HCoV-229E. DHA was found to enhance the antiviral effect of VPA, reducing the IC50 for viral replication inhibition. Combinations of VPA and DHA showed substantial antiviral synergy. Testing on SARS-CoV-2 revealed that VPA, DHA, and their combination inhibited viral replication, though preincubation did not improve effectiveness.
In the epidemiologic leg of the study, the Optum dataset analysis revealed a 14.9% COVID-19 positivity rate among over three million patients tested between 2020 and 2021. Higher percentages of Black and Hispanic patients were found in the COVID-positive group. Less than 25% of patients had serum VPA levels above the threshold for therapeutic levels. While univariate analysis showed no protective effect of VPA against COVID-19, multivariate models, accounting for comorbidities, indicated that VPA use was associated with 12–15% fewer emergency room (ER) visits, 17–45% fewer hospital admissions, 33–39% fewer cases requiring mechanical ventilation, and 14–16% fewer intensive care unit (ICU) admissions related to COVID-19.
Conclusion
In conclusion, the epidemiological findings of the study suggest that VPA use correlates with lower COVID-19 test positivity, ER visits, hospitalizations, ICU admissions, and mechanical ventilation needs, with effects persisting through the alpha and delta variants. Although the study does not confirm causality, combining VPA with DHA shows significant antiviral activity against coronaviruses, potentially due to the activation of antiviral genes. In the future, this combination may remain effective against new variants and reduce disease severity and spread. Further clinical research is warranted to confirm these findings and explore PUFA and HDAC inhibitor combination therapies for COVID-19.
Journal reference:
- Watson A, Shah P, Lee D, Liang S, Joshi G, et al. (2024) Valproic acid use is associated with diminished risk of contracting COVID-19, and diminished disease severity: Epidemiologic and in vitro analysis reveal mechanistic insights. PLOS ONE 19(8): e0307154, DOI: 10.1371/journal.pone.0307154, https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0307154