Can sweat reveal hidden signs of a heart attack? A new study finds that while plasma biomarkers provide crucial insights into post-heart attack inflammation, sweat biomarkers remain surprisingly static. Could this change the future of non-invasive cardiac monitoring?
 Study: Biomarkers of inflammation in sweat after myocardial infarction. Image Credit: ESB Professional / Shutterstock
Study: Biomarkers of inflammation in sweat after myocardial infarction. Image Credit: ESB Professional / Shutterstock
In a recent study published in the journal Scientific Reports, researchers assessed the potential of sweat as a noninvasive medium for detecting inflammatory biomarkers in patients with ST-elevation myocardial infarction (STEMI) post-percutaneous coronary intervention (PCI).
Background
Every 40 seconds, someone experiences a heart attack. Cardiovascular diseases, particularly myocardial infarctions, remain a leading cause of mortality worldwide. STEMI is a severe form of heart attack requiring immediate intervention. Following myocardial infarction, the body initiates an inflammatory response to repair damaged tissue.
While this process is essential for recovery, excessive or prolonged inflammation can contribute to adverse cardiac remodeling, increasing the risk of heart failure.
Traditionally, blood-based biomarkers such as high-sensitivity C-reactive protein (hs-CRP) and interleukin-6 (IL-6) are used to monitor inflammation. However, blood sampling is invasive and may not allow for real-time tracking.
Sweat, which can be collected non-invasively, is gaining interest as a potential alternative for monitoring physiological changes. Wearable technologies now enable continuous sweat biomarker analysis, opening avenues for personalized cardiovascular monitoring.
Despite this promise, little is known about the inflammatory biomarker profile in sweat following myocardial infarction. Further research is needed to determine whether sweat analysis can complement or replace traditional plasma biomarkers.
About the study
The present prospective observational study included 18 patients diagnosed with STEMI who underwent PCI and six control patients who underwent diagnostic coronary angiography without intervention. Participants were recruited from the Department of Cardiology at Örebro University Hospital, Sweden.
STEMI patients had their sweat and plasma samples collected at two time points: immediately post-PCI and at a follow-up visit 4-6 weeks later. Controls had samples taken at a single time point.
Sweat was collected non-invasively from the volar forearm using pilocarpine iontophoresis, followed by sample stabilization with a protease inhibitor. Plasma was obtained through venipuncture and processed for analysis. Both sample types were stored at -80°C until biomarker assessment. A high-throughput proximity extension assay (Olink Target 96 Inflammation panel) was used to quantify 92 inflammatory biomarkers in sweat and plasma.
Baseline patient characteristics, cardiovascular risk factors, and laboratory data were recorded. Statistical analyses included multiple linear regression models to assess differences in biomarker levels between time points and between STEMI patients and controls. Adjustments were made for confounding factors such as sex, hypertension, and diabetes. A significance threshold of p < 0.05 was applied.
Notably, the control group had a higher prevalence of pre-existing coronary artery disease (83% vs. 28%, p=0.049), which may have influenced biomarker differences between the two groups.
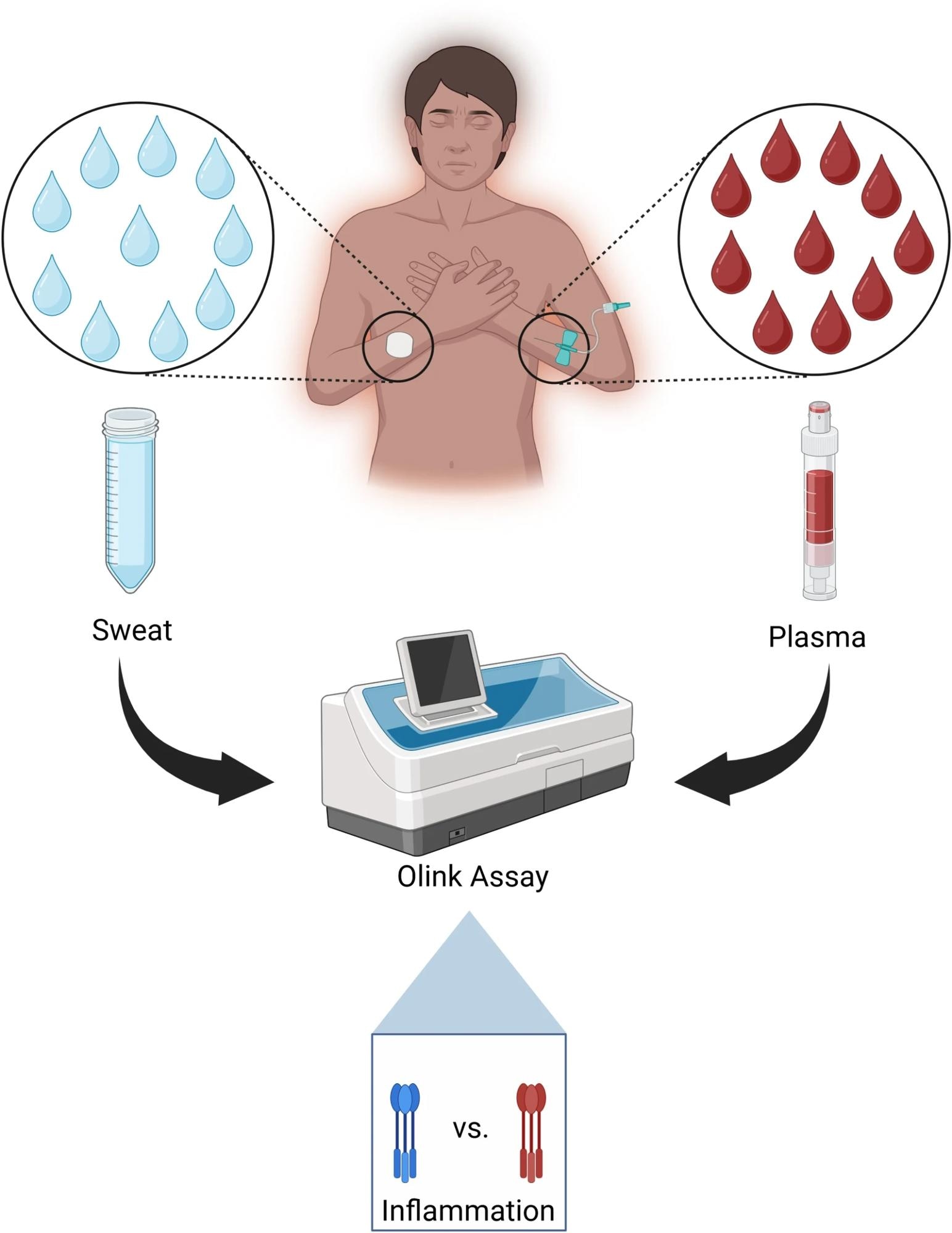
Sweat was collected non-invasively from the skin surface, and plasma was obtained from 18 STEMI patients immediately following PCI, and from 6 controls undergoing outpatient angiography without intervention. Ninety-two inflammatory biomarkers were analyzed using a high-throughput proteomic assay.
Study results
The STEMI group (83% male, mean age 59±6 years) and the control group (66% male, mean age 58±5 years) showed differences in pre-existing coronary artery disease prevalence (28% vs. 83%, p=0.049). The median door-to-balloon time in STEMI patients was 64 minutes, and the median hospital stay was three days.
Laboratory analysis of venous blood at the acute phase showed significantly elevated hs-CRP, high-sensitivity troponin-I (hs-TnI), and leukocyte counts in STEMI patients compared to controls (all p < 0.001).
At follow-up, these markers showed a significant decline (p < 0.001), suggesting resolution of acute inflammation. Additionally, N-terminal pro-b-type natriuretic peptide (NT-proBNP) was higher in STEMI patients than in controls (p=0.02).
In plasma, ten biomarkers showed significant changes from the acute phase to follow-up after adjusting for the patient group. IL-6 and hepatocyte growth factor (HGF) decreased significantly, indicating reduced inflammation. However, several plasma biomarkers, including interferon-gamma (IFN-γ), C-C motif chemokines (CCL19 and CCL25), Fms-related tyrosine kinase 3 ligand (FLT3LG), lymphotoxin-alpha (LTA), tumor necrosis factor ligand superfamily member 10 (TNFSF10), urokinase-type plasminogen activator (PLAU), and CUB domain-containing protein 1 (CDCP1), increased over time, reflecting a complex inflammatory response.
In sweat, biomarker levels did not show significant temporal variation. However, one biomarker, STAM binding protein (STAMBP), was significantly elevated in the sweat of STEMI patients compared to controls (p < 0.05), suggesting a potential role in acute myocardial inflammation. Plasma IL-6 levels were consistently higher in STEMI patients than in controls, confirming its role as a pro-inflammatory marker.
Conversely, plasma concentrations of TNFSF10, TNFSF11, and monocyte chemoattractant protein 2 (MCP-2) were lower in STEMI patients than in controls. The study suggests that lower TNFSF10 and TNFSF11 levels may reflect suppression of cell death to limit myocardial damage or depletion of susceptible cells during ischemia.
Conclusions
This study provides novel insights into the inflammatory biomarker profile in sweat following STEMI. While plasma markers such as IL-6 and HGF demonstrated significant dynamic changes post-infarction, sweat biomarkers remained relatively stable.
The significantly elevated levels of STAMBP in sweat suggest a possible role in myocardial inflammation, though its clinical relevance requires further investigation. The study also highlights that sweat may be less sensitive than plasma for detecting dynamic inflammatory changes following STEMI, at least with the biomarkers analyzed.
While sweat-based biomarker tracking holds promise, further studies are needed to determine whether it can provide complementary or unique information beyond traditional plasma biomarkers.
These findings could revolutionize cardiac care by paving the way for non-invasive, real-time inflammation monitoring using wearable sensors. However, larger cohort studies and alternative sweat collection techniques are required before clinical application to assess feasibility and sensitivity.
If proven effective, non-invasive biomarker tracking could enhance patient management, offering a more accessible, continuous, and painless method for monitoring inflammation beyond conventional blood tests.