From the Mediterranean diet to microbial metabolites, scientists reveal how maintaining gut health could be a powerful strategy to delay or prevent Alzheimer’s disease.
 Review: Microbial diversity and fitness in the gut–brain axis: influences on developmental risk for Alzheimer’s disease. Image Credit: Volodimir Zozulinskyi / Shutterstock
Review: Microbial diversity and fitness in the gut–brain axis: influences on developmental risk for Alzheimer’s disease. Image Credit: Volodimir Zozulinskyi / Shutterstock
In a recent review in the journal Gut Microbes, researchers at Gachon University, Republic of Korea, summarize more than 110 scientific publications to elucidate the role of the gut-brain axis in maintaining gastrointestinal health. The review defines and elaborates upon the axis, focusing on how optimal gut microbial health can prevent neurodegenerative conditions such as Alzheimer’s Disease (AD). It highlights both recent scientific advances in the field and gaps in the literature and how next-generation omics technologies can bridge these gaps, improving public health worldwide.
Background
The gastrointestinal (GI) tract hosts around 80% of the 100 trillion bacteria in the human body, containing hundreds or even thousands of times more bacterial genetic information than our own genomes. The gut microbiome predominantly includes Bacteroidetes and Firmicutes, alongside smaller populations of Actinomyces, Proteobacteria, Verrucomicrobia, and Fusobacterium, forming a unique ecosystem that governs host health.
Research emphasizes the importance of microbiome-host communication, achieved via chemical signaling compounds called quorum-sensing molecules (QSMs). These QSMs are crucial to the microbiota-gut-brain axis (MGBA), but can also promote virulence in pathogens like Pseudomonas aeruginosa. Germ-free animal models show that eliminating GI microbiota and QSMs leads to neurodevelopmental defects, abnormal gastric motility, and weakened immunity, highlighting their role in digestion and overall health.
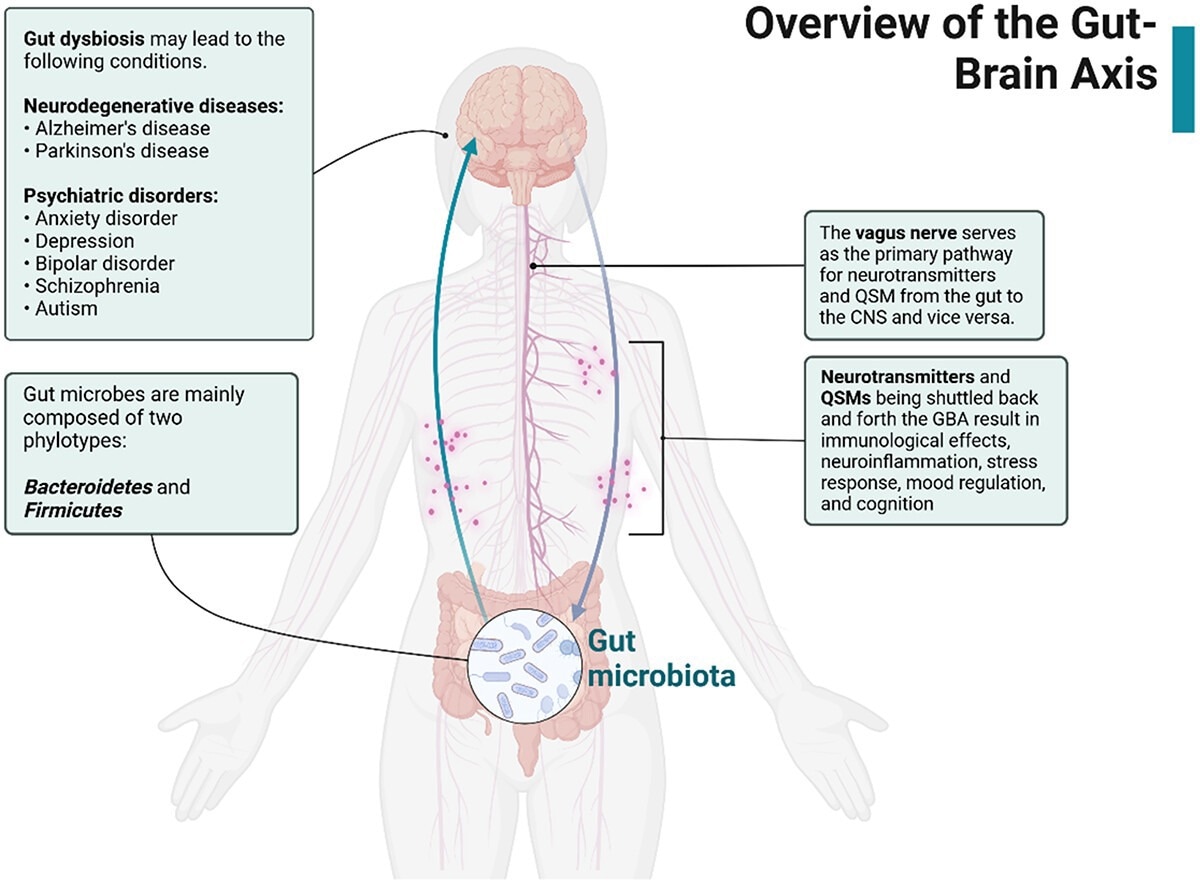
Overview of the gut–brain axis. Gut microbes release neurotransmitters and QSMs that travel through the vagus nerve and influence the CNS. In the event of gut dysbiosis, neurodegenerative disorders may arise as a result of the stress response, immunological response, and neuroinflammation. Created with BioRender.com.
Gut-brain communication
The MGBA links the central nervous system (CNS), digestive cells, enteric nervous system (ENS), and gut microbes, mediating bidirectional communication through various amines and peptides. While complex and not fully understood, animal models have shown their importance in maintaining homeostasis and well-being.
Gut dysbiosis, alterations in microbial populations, has been linked to anxiety-like behaviors, reduced sociability, cognitive issues, and atypical stress responses. Vagotomy studies underline the vagus nerve’s role in transmitting signals between the ENS and CNS.
QSMs influence gene expression in both microbes and hosts, facilitating communication and suppressing harmful bacteria—yet some enable pathogenic traits like biofilm formation. These molecules regulate microbiome behavior and critical processes like antimicrobial production and symbiotic balance. Certain QSMs, like short-chain fatty acids (SCFAs) and indoles, interact with host cells to influence immunity and neurological function.
“This interference with the development of other microorganisms through quorum-quenching enzymes and antagonistic autoinducer molecules was notable. It highlighted the intricacies of microbes and dynamic communication networks, which employed various strategies to gain a competitive edge within their communities.”
Endocrinology
While some QSMs aid in microbe-host communication, most function between bacterial populations. Key communicative molecules between the gut and host include serotonin, GABA, and dopamine.
Shifts in microbiome composition can hinder the synthesis of these essential molecules, contributing to depression and anxiety in both animal and human studies. Neuromodulators affect brain health through direct interaction with GI receptors or by entering the bloodstream and crossing the blood-brain barrier.
Dysbiosis and advances in molecular techniques
Sequencing technologies like proteomics, transcriptomics, and metagenomics have expanded our understanding of the microbiome’s role in health.
Nutritional studies show that diets like the Mediterranean diet and intermittent fasting (IF) support gut health, while Western diets promote microbial imbalances and increase risks for inflammation, neurological disorders, and cancer. IF has shown promise in reshaping gut communities and reducing Alzheimer's-associated amyloid-beta plaques.
“Gupta et al. developed the Gut Microbiome Health Index (GMHI), an algorithm predicting disease likelihood from microbial composition. From 4,347 human stool samples, 50 beneficial microbial species were identified. An updated GMHI 2 included 8,069 samples from 54 global studies, refining disease-specific predictions.”
Long-term dietary research shows that healthy diets like the Mediterranean diet and IF can suppress neuroinflammation and reduce Alzheimer’s disease risk. In contrast, Western diets may worsen disease progression by impairing the blood-brain barrier and promoting inflammation.
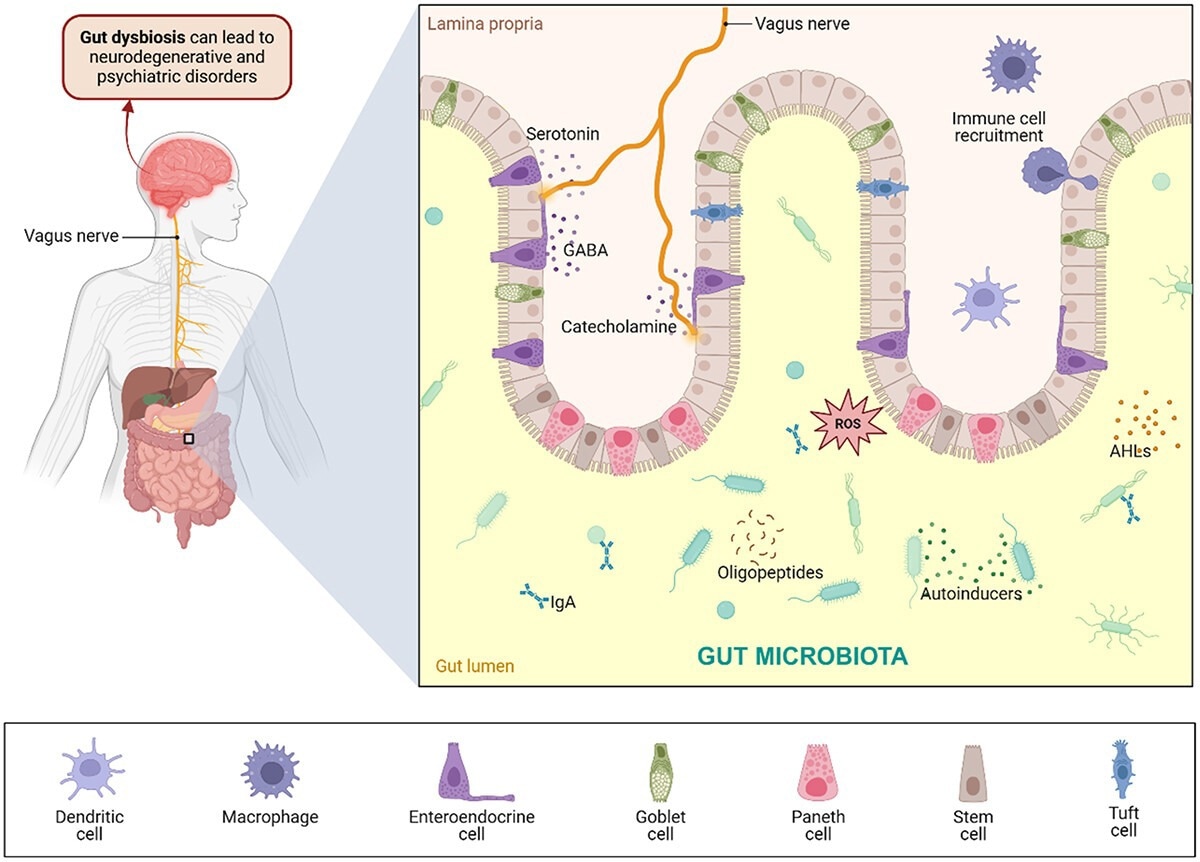
Cross-kingdom signaling in the GBA. The gut microbiota releases several neuromodulators, such as neurotransmitters, oligopeptides, and other metabolites, interacting with epithelial and immune cells. Specialized cells lining the intestinal epithelium maintain intestinal homeostasis, form a mucus barrier, and facilitate the passage of material through the intestines. Neurons also recognize neurotransmitters, which travel through the vagus nerve to the brain. Gut dysbiosis resulting from imbalanced gut microbial populations leads to immunological effects and neuroinflammation, possibly resulting in neurodegenerative and psychiatric disorders. Created with BioRender.com.
Conclusions
The MGBA is vital to health, influencing risks for various diseases. As understanding gut bacteria-host interactions deepens, new clinical interventions may emerge to combat the growing burden of non-communicable and infectious diseases.