Introduction
Whenever a sample is measured, the quality of the reading cannot exceed that of the measurement. Thus, it is vital to have a control on the sources of error that negatively impact the measurement or maintain them within defined limits. Errors can have technical causes or may be caused due to improper handling. Incorrect handling errors for photometric quantification includes sample concentration being too high or too low, wrongly programmed method factor, or insufficient sample mixing.
Handling errors are relatively easy to detect and thus can be corrected, but detecting technical errors involves making comparative measurements with materials of certified reference, and usually requires adjustments of the particular device for correction.
Clearly defined limits are given for devices developed by Eppendorf. Technical errors occurring within these limits do not alter the device’s accuracy. The limits also include the calibration medium, the measurement, and the methodical errors of the device.
In order to ensure that the limits are observed, the device should be periodically inspected. Regular inspection of measuring equipment is carried out by labs, which follow a quality management system, for example ISO 9001, ISO 17025, GMP, and GLP.
For aiding comparative measurements, Eppendorf provides test filter sets, which can be used as certified reference materials. The reference standards of the National Institute of Standards and Technology (NIST®) are the basis on which the filters are based upon. Wavelength and photometric precision of Eppendorf Photometers are tested with the help of the filter sets. The trueness and the accuracy of the photometer can be evaluated by comparing each filter’s measured value with the limiting value.
A standard reference photometer is used for validating the detection ranges of individual filter sets, which are supplied with the BioSpectrometer basic, BioPhotometer plus, BioSpectrometer kinetic, BioSpectrometer fluorescence, and the BioPhotometer D30. The BioSpectrometer kinetic and BioSpectrometer Basic share a common filter set.
Theoretical Background
ISO 3534-1 defines the meaning of terms related to the testing of devices. Figure 1 shows the links between some basic terms since several terms are frequently used in the literature in a rather unclear manner. There are two types of technical errors that affect the measuring result in a significant manner - systematic errors and random errors.
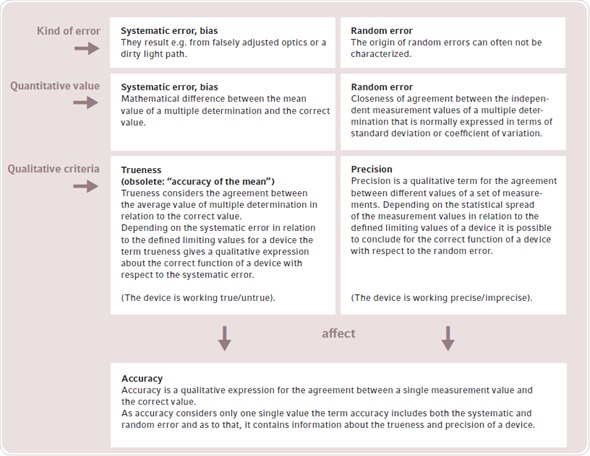
Figure 1. Explanation of the terms trueness, precision and accuracy according to the stipulations of ISO 3534-1. Conceptually, it can be differentiated between the type of error, the mathematically quantified dimensions of the error and the qualitative description of the function of a device. In contrast to trueness and precision, the term accuracy is hardly used when testing the functionality of a device and is only listed for the sake of completeness.
Systematic errors are caused by defective parts like incorrectly adjusted optics or interfering influences such as a dirty light path, and can lead to measurement result deviation from the real value. These errors can be detected by making comparative measurements with reference materials such as secondary UV-VIS-filter (Figure 1).
The mean value obtained from the measurement series is evaluated against the correct value, after a multiple determination is performed with the reference material. The quantitative value of the systematic error is calculated by the difference between the correct value and the mean value. If the systematic error is within the defined limiting values, a qualitative statement of trueness is made on the correct functioning of the device. The cause of “random error” cannot be easily characterized or avoided. Therefore, accuracy of the measuring device (Figure 1) and spread of quantified values are affected by random errors. Figure 2 graphically explains the link between precision and trueness in relation to accurate and consistent measurements.
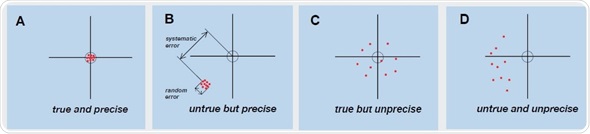
Figure 2. Graphic representation of the connection between trueness and precision when testing measuring devices. Individual measured values are shown as points. The middle of the cross of the axes represents the correct value, while the circle represents the permitted limits for the systematic error. In Fig. B, the deviations representing the systematic and random error are also shown for additional clarity.
Figure 2A shows that all measured values and their mean value are within the previously defined limits and exhibit only slight scattering. It can be stated that this measuring device is providing exact and reproducible measurement readings with a high level of precision and trueness, if it is assumed that the random error also falls within the limits (data not shown). Figure 2B shows the measurement series which is based on a systematic error. Due to this, all quantified values are deferred by the same amount, and fall beyond the limits. Figure 2A also shows that the individual measured values do not vary much from each other, thus showing that the device is providing false, though accurate measurement results. Figure 2D depicts high random and systematic errors in the distribution of the quantified values. The device shows both poor precision and poor trueness. Reproducible, accurate, and exact measurements can be obtained only with a device for which precision and trueness fall within the defined limits. The BioSpectrometer or BioPhotometer should be tested by the technical service for performing according to the specifications when the Photometer test results explained in the short instructions resemble the pattern in Figures 2B-D.
Short instructions for Performing the Photometer Test on the Eppendorf BioSpectrometer® and Eppendorf BioPhotometer®
General Information
- A temperature of approximately 20°C is essential for measurements to be carried out.
- The secondary UV-VIS-filter set is needed for performing the photometer test. The cuvette shaft holds the filters. Specific filter sets for the BioSpectrometer and BioPhotometer are needed
- Stickers should face the user. Test filters should be devoid of dust.
- The device should be connected to the printer for simplifying the process of documentation of the measured values.
- Forms are given along with each photometer, and can be used for transferring the limits present in the certificate in the filter box lid, and for entering the photometer test measured values.
Measurement Instructions for the BioPhotometer plus
- The BioPhotometer plus is switched on.
- The FUNCTION KEY is pressed, and the NEW MESUREMENT function is selected under the PHOTOMETER TEST in the menu by employing the ▲ and ▼ cursor keys (Figure 3A). The ENTER key is pressed to confirm.
- Five measurement protocols appear: SAMPLE 260nm and SAMPLE 280nm for finding trueness and precision of wavelengths 260 and 280nm. SAMPLE A1, SAMPLE A2 and SAMPLE A3 for finding metric trueness and precision at 230, 260, 280, 340, 405, 490, 550, 595 and 650nm.
- The suitable measurement protocol is selected with the help of the cursor keys and the selection is confirmed by pressing the ENTER key (Figure 3B). It is recommended that the five measurement protocols are carried out when the BioPhotometer plus is tested.
- The blank A0 filter is entered.
- The zero value adjustment is performed by pressing the ENTER key.
- The appropriate SAMPLE filter is entered.
- After the ENTER key is pressed, the BioPhotometer plus automatically performs 15 measuring cycles and determines coefficients of variation (CV) and mean values.
- The limiting values allowed for the relevant test filter are compared with CVs and measured values. The limiting values are present on the certificate in the filter box lid.
- The next measurement protocol is selected by pressing the ENTER or FUNCTION key.

Figure 3. (A) Function overview BioPhotometer plus; (B) Selection overview of the Photometer Test - BioPhotometer plus.
Measuring Instructions for the BioPhotometer D30 and BioSpectrometer
- The device is switched on.
- The function button on the device or the “softkey” function situated in the lower right corner of the display (Figure 4A) is pressed, if the main menu appears.
- The “Subgroup” and “Device calibration” are selected in sequence, and the “Photometer unit” or “Spectrometer unit” (Figure 4B) is selected. The method is opened by pressing the ENTER key.
- Two measuring protocols are displayed for selection (Figure 4C): (1) Wavelength frequency is checked. The precision and trueness are detected at wavelengths of 260, 280 and 800nm. The wavelengths of 260 and 280nm are checked for BioPhotometer D30. (2) The photometric accuracy is checked. The photometric precision and accuracy is checked with the filter SAMPLE A1, SAMPLE A2 and SAMPLE A3 at 260, 280, 320, 405, 550, 562, 595, 700 and 800nm; for BioPhotometer D30, the wavelengths are 260, 280, 320, 405, 550, 562, 595nm.
- The cursor keys are used to select the required measuring protocol, and the selection is confirmed by pressing the ENTER key.
- The filter A0 is inserted for the Blank measurement.
- The “Blank” key on the device is pressed.
- The correct SAMPLE filter is inserted into the cuvette shaft.
- “Sample” is pressed. The Bio-Spectrometer and the BioPhotometer D30 perform 15 measuring cycles, and determine the CV and the average values.
- The stated values on the certificate of the test filter set are compared with the CV and average values. The certificate is present in the filter box lid.
- The fluorescence unit can be inspected in the case of BioSpectrometer fluorescence. Two dynamic measuring ranges can be applied for fluorescence measurements according to the sample’s fluorescence intensity. The ranges depend on the defined ratio measuring intensity. This ratio can be validated for the fluorescence unit by using the reference filter (F1) (Figure 4D).
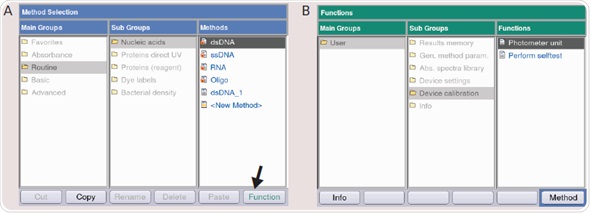
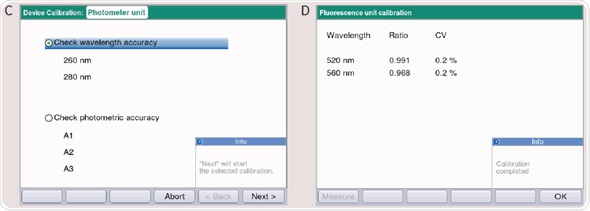
Figure 4. (A) Main Menu of the BioSpectrometer and BioPhotometer D30. By using the softkey “Function” (Arrow) you can enter the device functions incl. Photometer or Spectrometer test. (B) Functions overview of the BioSpectrometer and BioPhotometer D30: The photometer or spectrometer test is located in “Sub Group”/“Device calibration.” (C) Measuring protocols to determine the wavelength and photometric accuracy of the BioPhotometer and BioSpectrometer. (D) Checking the fluorescence unit on the BioSpectrometer fluorescence troubleshooting.
Troubleshooting
A trained service technician should test the BioSpectrometer or BioPhotometer if the measured values are beyond the specified range of limits following a repeat measurement. A maximum of two years guarantee is given for the validity of certified limits of the secondary UV-VIS-filter. The Technical Service can perform the recalibration of this secondary UV-VIS-filter.
Definitions
The maximum deviation of the average values from the values shown by the photometer in a specific measuring range is called as the photometric accuracy. The maximum deviation of the average values from the values shown by the photometer in a specific measuring range at one or more specific wavelengths is called as wavelength accuracy.
Acknowledgements
Produced from materials originally authored by Martin Armbrecht-Ihle and Lars Borrmmann from Eppendorf AG, Hamburg, Germany
About Eppendorf NA
Eppendorf NA, with headquarters in New York, are a subsidiary of Eppendorf AG (Hamburg, Germany) – manufacturer of laboratory instruments and consumables for the life sciences.
To make it easy for labs in the United States to benefit from Eppendorf quality, they provide total customer support and service: taking/tracking orders; product installation and training; application support; calibration services; general product maintenance and repair, and more!
Eppendorf's products
Eppendorf products are used in all types of life science research and testing settings – from basic laboratory applications to highly specialized cell and molecular biology applications. They are highly regarded for their quality design and performance – beginning with extensive research and development, adding state-of-the-art technology and ending with strict quality-controlled manufacturing are what make their products stand out from the rest. It is what has made them a brand you have been able to rely on for over 70 years.
Over the years Eppendorf has improved upon and added to their flagship products – pipettes and pipette tips, centrifuges and microcentrifuge tubes – to now include the most ergonomic liquid handling devices and automated pipetting stations, quiet centrifuges, fast and fully flexible thermal cyclers, sample-protecting deepwell plates, cell manipulation systems and microcapillaries, ultra-low temperature freezers, shakers, incubators and bioprocessing equipment.
Eppendorf's services
Customer Support Representatives are available M–F, 8:30 AM – 8:00 PM (EST).
Local Sales Representatives cover the entire US to give you the fastest best possible attention. They know their products – and better yet, they know your applications. Feel confident in their abilities to assess your needs and determine the best products and systems to give you the best results.
Field Specialists also cover the entire US to provide post-sales installation and training on their specialty systems and automation.
The Applications Hotline is manned by degreed scientists working in their own fully functional life science laboratory. They are on call and email-accessible to help you troubleshoot your application or answer any technical question you may have. The Eppendorf Services group provides a wide variety of support services such as pipette calibration, instrument maintenance programs, and expedited repair.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.