Introduction
The modification of laboratory animal genomes is carried out by micromanipulation of embryos or oocytes. The usual techniques of micromanipulation are: transcription activator-like effector nuclease (TALEN) into the cytoplasm into a pronucleus of early zygotes; or microinjection of nucleic acid constructs like larger bacterial artificial chromosomes (BAC), simple transgenes, and zinc finger (ZFN).
Such methods are known as cytoplasmic and pronuclear injection, respectively. ES cell transfer is another method, which involves genetically modified embryonic stem cells injection into embryos in 8-cell stage or blastocyst. One other technique is the intracytoplasmic sperm injection (ICSI) of sperms coated with nucleic acid (sperm-mediated gene transfer) or genetically altered sperms into oocytes, for generating genetically modified offspring.
All the aforementioned methods vary in the optimal injection angles, and differ in manipulators requirements and in the injection movement speeds. Therefore, most labs use adjustable and independently pre-fixed workstations for various injection technologies. Eppendorf AG has come up with TransferMan® 4r (Figure 1), a multifunctional manipulator, which is apt for all these applications.

Figure 1. Eppendorf TransferMan 4r micromanipulators.
TransferMan® 4r has many features such as DNA injection, cell transfer or ICSI, so as to simplify the workflow process. Intuitive and precise movement in all spatial dimensions during injection is enabled by the novel DualSpeed joystick. The workstation can withstand all external vibrations. The manipulator elements can be adjusted and installed quickly and easily. The motor modules’ positional changes suit all types of microscopes. Angle adjustment is easily accomplished; it is easy to set shallow angles of 0° (preferred for ICSI or pronucleus injection) or up to 45°.
Materials and methods
The following devices are required for performing the genetic engineering technique:
- two TransferMan® 4r micromanipulators (one for positioning the transfer/injection capillary and the second for moving the holding capillary)
- infrared laser for laser-assisted ES cell transfer, e.g. from Hamilton Thorne (USA) or OCTAX® (Germany)
- inverted microscope integrated with differential interference contrast (DIC), and 10x, 20x and 40x objectives
- CellTram® Air microinjector for holding the oocyte
- adapter for inverted microscope
- CellTram® vario microinjector for transferring the ES cells
- holding capillaries, for example Eppendorf VacuTips
- FemtoJet® microinjector for pronucleus injection
- ES cell transfer capillaries, e.g. Eppendorf Piezo Drill Tips ES, TransferTips ES
- injection capillaries from BioMedical Instruments
- Eppendorf Microloader™ for filling the injection capillaries
- operation of the Micromanipulator TransferMan 4r
The TransferMan 4r micromanipulator as well as the joystick and the functional soft keys can be operated in a simple manner (Figure 2). The saving of three capillary positions, setting of a vertical limit, and avoiding capillary breakage are enabled by choosing the “Cell transfer” application mask. The “DNA injection” application mask allows temporary deactivation of the Y motor, and allows for storing the capillary position.
The joystick allows easy control of the microcapillary in all three directions, and the TransferMan 4r can store up as much as five independent positions, based on the type of application mask selected.
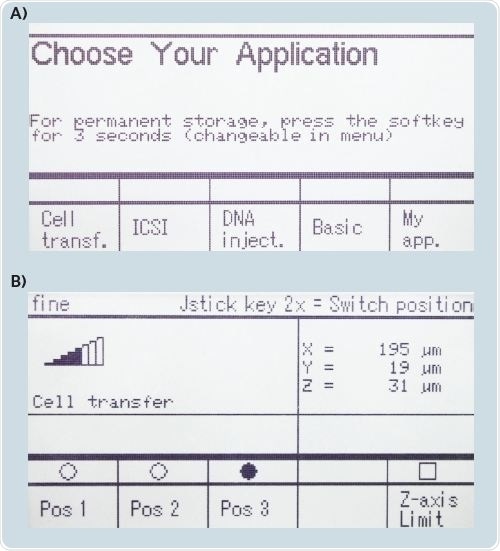
Figure 2. Display of the TransferMan 4r control panel: (A) Initial display: “Choose Your Application”; (B) “Cell transfer” application mask selected with function keys for position storage and Z-axis limit. The 4th softkey is user-definable e.g. for “Axial”, “Clean” or other functions.
The standard movement mode of the joystick allows direct transmission of the hand movement to the microcapillary. The joystick can be activated in the dynamic mode when the highest deflection of the radius of actual path has been attained, so as to allow the needle to move in the preferred direction with increasing speed (Figure 3). The use of the joystick and the user-definable application mask quickens the workflow, and reduces the time needed for the samples to be held under the microscope’s light beam.
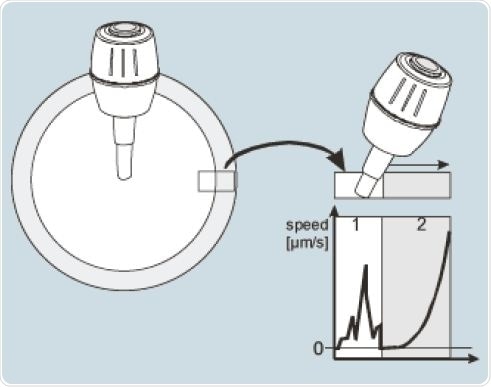
Figure 3. Principle of the DualSpeed joystick with direct (1) and dynamic deflection (2).
Preparation of cells, embryos and injection samples
Different organisms can be utilized for cytoplasmic or pronuclear injection; similarly, many laboratory models exist for ES cell transfer. The procedure for the injection methods is similar for different species, though this article concentrates on mouse model generation.
Preparation of the workstation for pronuclear (and cytoplasmic) injection
One of the TransferMan 4r micromanipulators is employed for injection capillary movement, and the other is used for holding capillary movement (Figure 4).
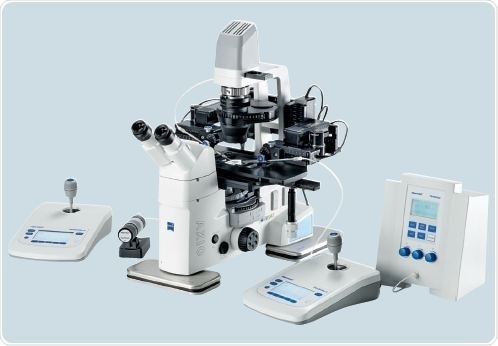
Figure 4. Workstation set-up for microinjection of nucleic acids into the zygote: TransferMan 4r, CellTram Air and FemtoJet in combination with Zeiss® Axio Vert.A1 microscope.
A holding capillary and a CellTram Air microinjector are employed for holding the embryo. Tapered microinjection capillaries and the programmable microinjector FemtoJet® are employed for nucleic acid injections. As the microcapillaries for pronuclear injection are straight, the injection angle needs to be flat to the extent possible. Different injection chambers are available which act as a microenvironment for the zygote during the process of injection. Here, an injection chamber based on required specification was used.
A drop of M2 medium is placed on a homemade injection chamber. After placing 10-20 zygotes in an area of the drop, it is covered with oil. The injection is activated by hand or foot control of the FemtoJet. The continuous flow option is recommended for cytoplasmic and pronuclear injections, since the pronuclei have different sizes, and the pronuclei swelling for the zygotes are realizable in individual injection time.
Moreover, the combined pronculei and cytoplasmic injection can be realized in one step if the continuous flow option is utilized. Empirical determination of the basic settings of the injection pressure (pi) and the compensation pressure (pc) are needed, based on the injection capillaries inner diameters. The injection and holding capillaries are accommodated into the capillary holder and aligned, prior to the injection.
The “Clean” function should be activated for clearing clogs in the capillary, before the injection. For optimization of the injection workflow under the microscope, the following procedure was adopted (Figure 5).
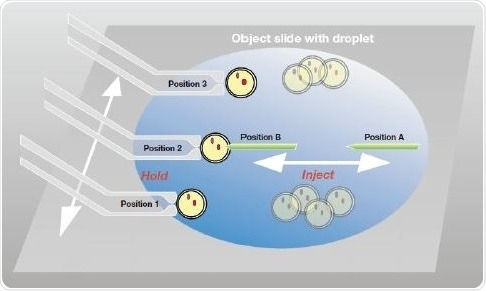
Figure 5. Storage of positions for nucleic acid microinjection into zygotes. For the holding capillary (left side) three different positions are stored for uptake, injection and collection of the zygotes. For the injection capillary two positions (injection and parking) are set.
On the holding side, when the capillary reaches the area in which the uninjected zygotes are placed in the M2 drop, “Position 1” of the TransferMan 4r storage function is activated. The CellTram Air microinjector along with the fixed VacuTip holding capillary easily takes up a zygote, which is shifted to a central location for nucleic acid injection.
This position is saved as “Position 2.” The zygote is then shifted to another location in the M2 drop, after injection, for separately collecting the injected zygotes and the uninjected zygotes. This capillary location is stored as “Position 3.” On the injection side, the injection capillary is brought close to the fixed zygote (“Position B”), which is to be injected; the parking position is denoted as the “Position A.”
Preparation of the workstation for ES cell transfer into blastocysts or morulae
Two TransferMan 4r devices are employed for the transfer and holding capillaries control, during the shifting of ES cells into the embryos (Figure 6).
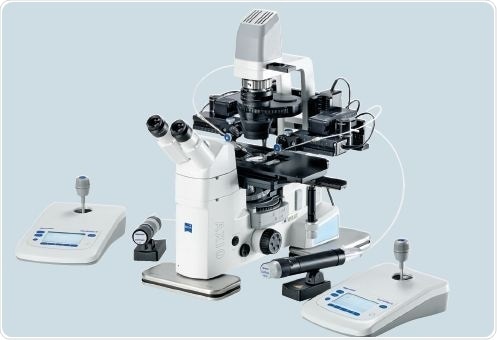
Figure 6. Workstation setup for ES cell transfer into blastocysts: two TransferMan 4r, CellTram Air and CellTram vario in combination with Zeiss Axio Vert.A1.
The VacuTip holding capillary and the CellTram Air hold the morulae or blastocysts. The TransferTip (ES) capillary and the CellTram vario enable the transfer of the ES cells. Blunt end tips such as Drill Tips ES, rather than spiked needles, can be utilized for laser-assisted transfer or piezo-assisted transfer. The transfer and holding capillaries are fixed in the capillary holder.
Mineral oil is filled in the transfer capillary, by rotating the CellTram vario wheel. The two capillaries are aligned at the base of the injection chamber. After placing a drop of M2 medium in the injection chamber; 20 early embryos are shifted into a location in the drop. In addition, ES cells, numbering a few hundred, in the medium are pushed into the injection chamber.
The following procedure was adopted for optimizing the workflow under the microscope (Figure 7).
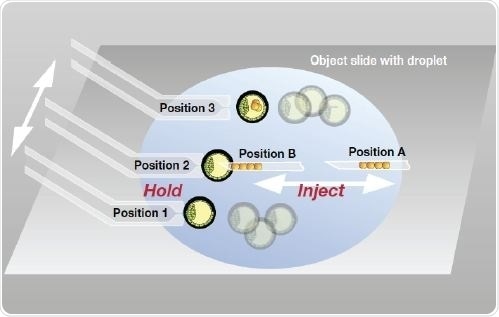
Figure 7. Storage of positions for ES cell transfer into blastocysts. For the holding capillary (left side) three different positions are stored for uptake, injection and collection of the embryos. For the injection capillary two positions (injection and cell collection) are set.
On the holding side, “Position 1” is saved, if the capillary reaches the area in which the uninjected embryos are kept. The CellTram Air microinjector along with the fixed VacuTip holding capillary easily takes up an embryo that is subsequently shifted to a central location for ES cells injection.
The current capillary position is saved as “Position 2.” The embryo is shifted to another location in the M2 drop, after the injection, for separately collecting the injected zygotes and the uninjected zygotes. This capillary location is stored as “Position 3.”
Under phase contrast and 20x magnification, the ES cells’ quality can be analyzed, and the cells can be selected based on their shape and size. For injecting blastocyst, ES cells, numbering 15 to 20, are collected into the capillary along with a small quantity of medium, and positioned proximal to the tip opening.
An embryo is held tightly to the holding pipette in the injection position, by utilizing the CellTram Air. After placing the blastocysts, the injection needle tip is aligned to a focal plane which is same for the blastocyst’s equator. This effect is realized by slowly touching the embryo surface with the tip so as to find the right plane.
The loaded injection capillary is moved into the blastocyst cavity, in one continuous movement, so as to transfer the cells into the cavity. Care should be taken to avoid pushing of lysed cells or oil bubbles into the blastocyst. Post-injection, the capillary is carefully taken out from the embryo.
Perforation of the zona pellucida by a laser enables the microcapillary to be penetrated into the embryo, thus allowing for the ES cells to be injected into 8-cell stage morulae or embryos. The injection capillary is inserted through the zona pellucida perforation, and about five to eight ES cells are introduced into the space of the perivitelline. The needle is withdrawn slowly, and the embryos are released from the holding capillary.
Results and Discussion
Pronuclear Injection
As regards to pronuclear injection of DNA, it is useful to shift the injection capillary with electric motors in a vibration-free motion. An exact movement of the injection enables low mortality. For this purpose, the Eppendorf TransferMan® NK 2 was employed for regular injections.
The same injection results obtained by utilizing the TransferMan NK 2, were also obtained when the TransferMan 4r was employed for many transgenic projects for pronuclear injection (Table 1).
Table 1. Results of pronuclear injection experiments performed during a time period of four weeks.
|
Manipulator
|
Zygotes injected
|
Lysis rate of injected zygotes
|
Injected zygotes transferred
|
Founder born
|
|
TransferMan NK2
|
1024
|
23.4%
|
784
|
5
|
|
TransferMan 4r
|
1335
|
18.4%
|
1090
|
5
|
By utilizing an extra 10° adapter for shallow injections in tandem with the TransferMan 4r and the TransferMan NK 2, the injection angle can be easily adjusted to about 5° so as to carry out ultra-low injections.
ES Cell Injection
Classical blastocyst injection has been in use for a prolonged period in the author’s facility. This technique results in producing chimeric animals having variable ES cell contribution. During the transfer of ES cells, the injection movement requires a straight and dynamic force, which in common practices, is realizable by a hydraulic controlled system that enables direct hand movement transmission from the joystick to the glass capillary.
For ES cell injection with the TransferMan 4r, the 8-cell embryo injection assisted by laser was first tested, and later on blastocyst injection, so as to determine the performance of the manipulator. Although being a motor-driven system, the TransferMan 4r works directly and dynamically, similar to a hydraulic or mechanical system. For efficient blastocyst injections, impetus and short injection capillary movements are needed; this effect is enabled by the joystick transmission and the motors. The workflow can be accelerated by utilizing the dynamic movement mode.
Conclusion
In conclusion, employing the TransferMan 4r for ES cell injections into the blastocysts and into 8-cell embryos were a success, and the results are comparable to those obtained with a hydraulic system.
Acknowledgements
Produced from materials originally authored by Ronald Naumann from the Transgenic Core Facility, Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany.
References
- Nagy, A., Gerstenstein, M., Vintersten, K., Behringer, B.: Manipulating the Mouse Embryo, a Laboratory Manual. Third edition (2003) Cold Spring Harbor Laboratory Press.
- Davies B, Davies G, Preece C, Puliyadi R, Szumska D, Bhattacharya S. Site specific mutation of the Zic2 locus by microinjection of TALEN mRNA in mouse CD1, C3H and C57BL/6J oocytes. PLoS One. 2013;8 (3):e60216. doi: 10.1371/journal.pone.0060216.
- Moreira PN, Pozueta J, Giraldo P, Gutiérrez-Adán A, Montoliu L.: Generation of Yeast Artificial Chromosome Transgenic Mice by Intracytoplasmic Sperm Injection. Methods Mol Biol. 2006; 349:151-61.
- Pease, Shirley; Saunders, Thomas L. (Eds.) Advanced Protocols for Animal Transgenesis. An ISTT Manual (2011), XV, Springer Protocols Handbooks.
About Eppendorf NA
Eppendorf NA, with headquarters in New York, are a subsidiary of Eppendorf AG (Hamburg, Germany) – manufacturer of laboratory instruments and consumables for the life sciences.
To make it easy for labs in the United States to benefit from Eppendorf quality, they provide total customer support and service: taking/tracking orders; product installation and training; application support; calibration services; general product maintenance and repair, and more!
Eppendorf's products
Eppendorf products are used in all types of life science research and testing settings – from basic laboratory applications to highly specialized cell and molecular biology applications. They are highly regarded for their quality design and performance – beginning with extensive research and development, adding state-of-the-art technology and ending with strict quality-controlled manufacturing are what make their products stand out from the rest. It is what has made them a brand you have been able to rely on for over 70 years.
Over the years Eppendorf has improved upon and added to their flagship products – pipettes and pipette tips, centrifuges and microcentrifuge tubes – to now include the most ergonomic liquid handling devices and automated pipetting stations, quiet centrifuges, fast and fully flexible thermal cyclers, sample-protecting deepwell plates, cell manipulation systems and microcapillaries, ultra-low temperature freezers, shakers, incubators and bioprocessing equipment.
Eppendorf's services
Customer Support Representatives are available M–F, 8:30 AM – 8:00 PM (EST).
Local Sales Representatives cover the entire US to give you the fastest best possible attention. They know their products – and better yet, they know your applications. Feel confident in their abilities to assess your needs and determine the best products and systems to give you the best results.
Field Specialists also cover the entire US to provide post-sales installation and training on their specialty systems and automation.
The Applications Hotline is manned by degreed scientists working in their own fully functional life science laboratory. They are on call and email-accessible to help you troubleshoot your application or answer any technical question you may have. The Eppendorf Services group provides a wide variety of support services such as pipette calibration, instrument maintenance programs, and expedited repair.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.