Patients are administered parenteral drugs usually by means of injections, and therefore these drugs must be free of visible particles. To ensure this, the USP <788> Particulate Matter in Injections test is employed for measuring the size and count of sub-visible particles in parenteral drugs, as shown in Figure 1. This test requires counting of particles on a filter by using microscopy and a light obscuration particle counter.
Figure 1. Parenteral drugs
Requirements for complying with USP <788> testing
A light obscuration sensor, and appropriate sample feeding device, a sampler, and a sensor are the tools required for a light obscuration instrument employed to comply with USP <788> testing.
The sensor needs to be calibrated for size at several points and should be then tested and validated for resolution and count efficiency, respectively. The concentration of the particles to be counted needs to be less than the concentration range. It is necessary that the dynamic range contains the smallest particle size to be quantified.
For the test, the accuracy of the sample volume needs to be within 5% of the appropriate sample volume. Particle concentration needs to be greater than 10 and 25 µm for reporting purposes.
AccuSizer 780 SIS Syringe Injection Sampler

Figure 2. AccuSizer 780 SIS Syringe Injection Sampler
Figure 2 shows the AccuSizer 780 SIS Syringe Injection Sampler from Entegris this instrument is specifically designed for conducting USP <788> particle testing, and satisfies and surpasses all USP <788> requirements.
For USP <788> testing, a high resolution particle sizing sensor LE400 is utilized. The sensor measures from 0.5 to 400µm at concentrations up to 9000 particles/mL, and features a patented optical design. Each sensor is checked for count efficiency at 15µm, and is calibrated with 10 particle size standards across the entire range.
Unlike conventional contamination monitoring systems that feature 6 or 8 size classification channels, the AccuSizer high resolution counter has more than 512 size channels. The AccuSizer is integrated with the high resolution particle sizing sensor.
While the resolution needed is 10% in USP <788> particle testing, the resolution offered by the AccuSizer sampler is 5% which is better than the requirement. Figures 3 and 4 illustrate the results when few and many size channels are used.
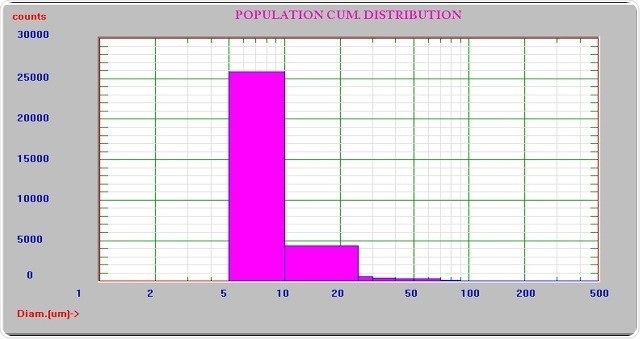
Figure 3. Results using few size channels
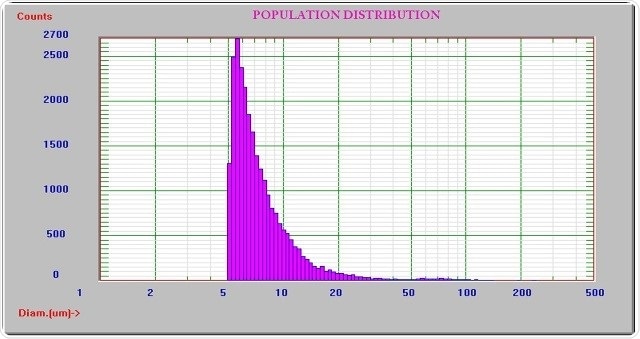
Figure 4. Result using many size channels
By using the new FX series sensors, the concentration limit is increased up to 400 times as compared to that of conventional light obscuration sensors, and the lower size limit is extended to 0.15µm.
Fully automated single-particle sizer
The first fully automated single-particle sizer, AccuSizer 780 SIS provides highly precise volumetric sample aliquots for many applications such as protein aggregation studies and USP <788> testing at minimum sample volume (< 1 mL).
The sampler doubles up as a particle counter and a high resolution particle sizing instrument, and offers high resolution particle size distributions without any assumptions concerning the distribution shape.
The measuring instrument reports raw data, which contains particle counts vs. size. Using simple statistics, these data points are changed by the software into other practical weighted distributions such as volume, area, number, volume/surface, etc., and the software also offers precise statistical data traceable to the raw data.
By combining high resolution particle size and contamination monitoring, the single instrument helps users to determine the cause of the contamination by providing a high resolution size image of contaminants.
If the contaminants are in a single size range, the assumption is that they were caused by a failure in the process and not because of a natural phenomenon that generally produces particles with a wider size distribution.
Quantifying sub-visible particles
In majority of applications, the presence of even a few large agglomerates can significantly affect the success and failure of a product. The presence of a few large particles in certain pharmaceutical products can considerably alter the content uniformity of a dose. In the case of emulsions, sample instability may be indicated by the appearance of a larger peak.
The AccuSizer 780 SIS sampler is capable of counting and sizing low levels of aggregate particles that are several standard deviations away from the main peak of the distribution. This information has been used by customers to make new formulations that are also more stable.
The AccuSizer is designed to detect the smallest difference in size distribution. This feature has allowed customers to develop better products and thus prevent potentially dangerous releases.
AccuSizer with CFR 21 Part 11 compliant software
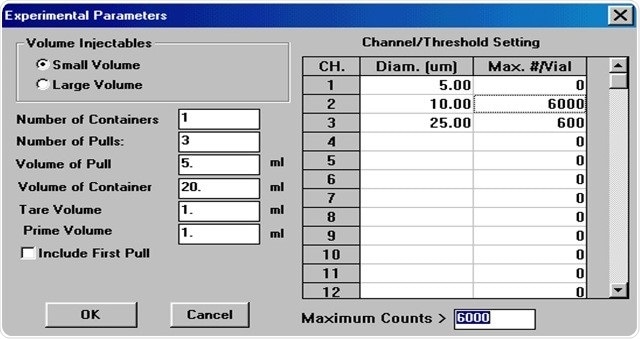
Figure 5. AccuSizer software setup for USP <788>
The AccuSizer 780 SIS sampler is quite flexible and is adapted to work with USP <788> particle testing. It is available with CFR 21 Part 11 compliant software, as shown in Figure 5. For experimental parameters, countless number of user-defined recipes is included in the instrument with simple tables.
The size pass/fail criteria and the types of samples are defined by these tables. For investigating the samples, users can either use their own internal tests or use the standard USP <788>. This flexibility comes handy when working on internal standards that satisfy or exceed the USP criteria.
Two customer interface modes are provided in the AccuSizer 780 SIS sampler — the high resolution particle sizing mode with up to 512 logarithmically-spaced channels, and the standard contamination monitoring mode with up to 32 user-defined channels.
All the sample analyses are saved and channels are combined into the user-defined channels in the high resolution mode. Customers can toggle between the two modes by just clicking the mouse; they can also change the user-defined channels and again measure the results without the need to rerun the samples.
Additionally, users can define up to 32 pass/fail parameters based on size and particle counts higher than that of size, thanks to the integrated pass/fail criteria tables in the software. The following table is based on the USP <788> criteria of 10 and 25 µm with regard to small volume injectables.
Table 1. USP <788> pass/fail criteria
|
|
>10 µm
|
>25 µm
|
|
Small volume injections
|
6000
|
600 per container
|
|
Large volume injections
|
25
|
3 per ml
|
Conclusion
Entegris’ AccuSizer 780 SIS Syringe Injection Sampler is designed to satisfy and surpass all requirements of USP <788> particle testing. It is well-suited for the pharmaceutical sector such as USP <788> large and small volume injectables.
About Entegris
For over 35 years, Entegris has been committed to helping customers find solutions to their particle sizing problems. We offer products with unique capabilities that can size particles from single digit nanoparticles to particles that are thousands of microns in diameter. Whether you need a particle size distribution or to find that needle in a hay stack, our team of engineers and sales staff are available to provide the product knowledge and scientific expertise necessary to solve your sizing issues.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and iform site visitors interested in medical research, science, medical devices and treatments.