Several new pharmaceutical preparations, including new vaccines and protein-based drugs, have been commercially available for the past few years. Safety and efficacy of all types of pharmaceutical preparations are largely ensured by Particle Size Analysis. Keeping drug safety in mind, the US Pharmacopeia (USP) contains many particle sizing methods.
In comparison to other particle sizing methodologies that are commercially available, the single particle optical sensing (SPOS), also known as light obscuration, provides unmatched sensitivity, resolution, and detail, and also offers quantitative concentration data. In order to set the usable limits for contamination, it is important to quantify the amount of particles.
Capabilities of SPOS technique
The large diameter tails of lipid globule size distribution can be statistically analyzed by employing the SPOS technique. Here, the target particles arise due to inherent instability of the emulsions, and not from contamination.
Larger particles are formed when smaller particles combine together, thus giving rise to dosage and safety concerns. This formed the basis for adding the SPOS technique into USP-729. Even trace amounts of the globule tails can be quantitatively measured by SPOS.
High resolution and accurate counting and sizing of particles are ensured by using SPOS technology. However, the need for high dilution to avoid coincidence and a large lower limit are the drawbacks of this technology.
Focused Light Obscuration technology
The new technology, Focused Light Obscuration, combines a smaller detection zone with advanced pulse analysis techniques in order to extend the lower size limit of detection to 0.15µm.
Upon focusing the beam of detection to a fine point, particle sizing and counting can be realized at concentrations of up to 10,000,000 particles/mL as compared to that of 10,000 particles/mL when standard SPOS is employed.
Figure 1 illustrates a particle size distribution from a commercial form of this technology known as AccuSizer FX-Nano from Entegris.
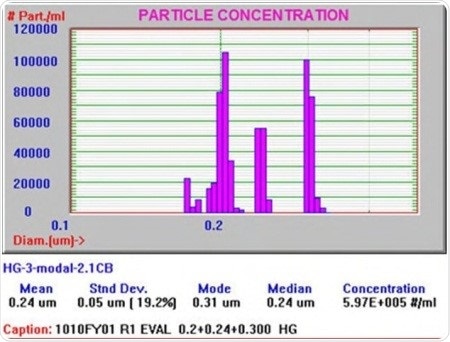
Figure 1. Particle size distribution from Entegris’ AccuSizer FX-Nano.
The sample was made up of a mixture of three polystyrene latex size standards from Duke Scientific in sizes of 200nm, 240nm, and 300nm. The final particle size distribution revealed all the three sizes.
The level of detail offered by this result cannot be achieved with static and dynamic light scattering techniques that are used to size nano-particles and to determine the mean diameters of samples including lipid emulsions. Moreover, light scattering techniques also lack the resolution needed to view the peaks individually and do not offer detailed concentration data.
Another mixture of polystyrene latex standards with sizes of 300nm, 340nm, 400nm, 500nm, and 600nm is shown in Figure 2. This result was achieved at a lower gain, which led to the measurement being more sensitive to larger particles. Such an informative result will be difficult to achieve through other particle size analyzers functioning in this size range.
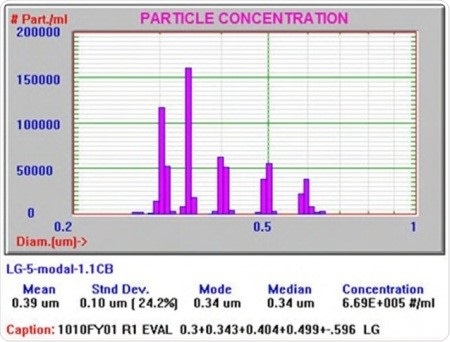
Figure 2. This result was obtained at a lower gain that made the measurement more sensitive to larger particles.
The Focused Light Obscuration technology was employed to analyze a host of injectable lipid emulsions in order to test its capabilities. The result from one sample is represented in Figure 3. This result is similar to what would have been obtained if a light scattering device had been used, with small differences in the large globule tail and particle size distribution (PSD), which other techniques will be unable to provide.
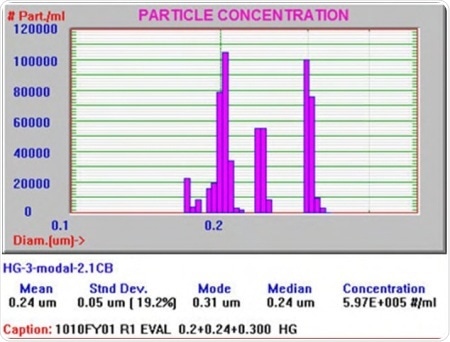
Figure 3. PSD of an injectable lipid emulsion using Focused Light Obscuration technique
Figure 4 presents an overlay graph comprising of the PSDs of many similar lipid emulsions including the one shown in Figure 3 (the blue curve). The occurrence of varying amounts of de-stabilization is shown by the PSDs. In all cases, the distribution extended out from the coarse side of distribution.
This may be expected if the particles underwent Oswald ripening, which could explain why the light scattering devices were not able to detect the onset of the de-stabilization of an emulsion PSD.
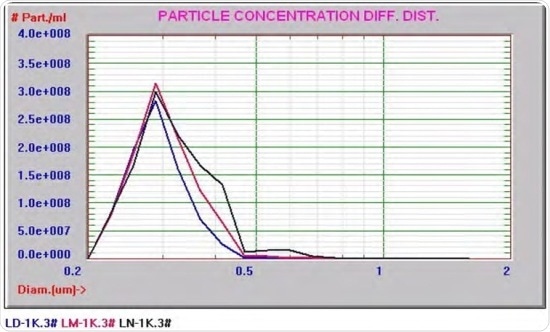
Figure 4. Overlay graph consisting of the PSDs of several similar lipid emulsions
It appears that there are slight overall mean changes at the beginning of the ripening process, and that the light scattering measurements observe only the mean value.
When the coarse side of the distribution extends outwards, this will be considered as a change in the mean diameter by light scattering devices because the algorithms that are used to deconvolute the scattered light signals are unable to detect the slight changes in the shape of a distribution.
Conclusion
Researchers have raised concerns regarding the state of knowledge of the PSDs in novel protein drug formulations, particularly on the safety risks related to the sub-visible protein particles derived from aggregation. These particles may have lower size than the 10µm limit specified by USP-788.
Also, the size of these particles could be less than the 0.5µm sensitivity of standard SPOS, thus necessitating a better understanding about the level of aggregation occurring in the new drugs. This issue could be addressed by Focused Light Obscuration technology that can count and size down to 200nm even at high particle concentrations.
About Entegris
For over 35 years Entegris has been committed to helping customers find solutions to their particle sizing problems. We offer products with unique capabilities that can size particles from single digit nanoparticles to particles that are thousands of microns in diameter. Whether you need a particle size distribution or to find that needle in a hay stack, our team of engineers and sales staff are available to provide the product knowledge and scientific expertise necessary to solve your sizing issues.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and iform site visitors interested in medical research, science, medical devices and treatments.