As larger size fat globules (>5 μm) may be trapped in the lungs and are also a sign that the emulsion is destabilizing, the size of the lipid droplets is crucial. Two analytical methods are required for the USP <729> test:
- Light obscuration to measure the large tails >5 μm
- DLS, or laser diffraction, to measure the mean and standard deviation of the distribution
The AccuSizer® is the preferred technique to measure the tails >5 μm, and the Entegris Nicomp® can be utilized to measure the mean size.
Introduction
By supplying essential vitamins and fatty acids, injectable lipid emulsions (Figure 1) have been clinically utilized for decades as an energy source for hospitalized patients. Intralipid, and other balanced lipid emulsions, supply essential fatty acids, an omega-6 fatty acid, linoleic acid (LA), alpha-linolenic acid (ALA), an omega-3 fatty acid.
The critical size characteristics of lipid injectable emulsions include the large diameter tail >5 μm and the mean droplet size. No single method can measure both parameters adequately, so two techniques exist in USP <729>:
Figure 1. Image Credit: Entegris Particle Sizing
Method I – light scattering method
Either laser diffraction (referred to as classic light scattering in the method) or dynamic light scattering (DLS) is employed to measure the mean size. The chi square parameter must be acceptably low and the intensity weighted mean diameter has to be <0.5 μm.
Method II – light obscuration method
The measurement of large globule content using single particle optical sizing (SPOS) using light obscuration or extinction techniques. The volume weighted percent above 5 μm must not be more than 0.05%.
Method I
The Nicomp DLS system shown in Figure 2 is the perfect system to utilize for Method I testing to establish the mean droplet size. The steps below should be followed in order to comply with the requirements set in USP <729>:
- Check the system performance with PSL standards at 100, 250 and 400 nm.
- The coefficient of variation (COV) has to be <10% of the reference values.
- Dilute the sample to an appropriate concentration and quantify the size at 90°. Verify that the chi square error calculation is at an acceptable low, and record the intensity weighted mean diameter. This value must be <0.5 μm (500 nm).

Figure 2. The Nicomp DLS system. Image Credit: Entegris Particle Sizing
Method II
The large diameter droplet tails are quantified by utilizing a light obscuration/extinction liquid particle counter, which uses the single particle optical sizing (SPOS) method, like the AccuSizer, as seen in Figure 3. Further guidance on utilizing this method can be found in USP<788> and USP <1788>.

Figure 3. AccuSizer APS. Image Credit: Entegris Particle Sizing
Verifying the sizing and counting accuracy of the light obscuration instrument should be carried out by utilizing two different size standards of ~5 and 10 μm (triplicate analyses per size). The average mean diameter on a number weighted basis should be within 10% of the expected value.
Set the upper size limit at 50 μm and the lower size limit at 1.8 μm. The volume-weighted result >5 μm (PFAT5) must be <0.05%. Vary the measurement time so that there is a factor of two difference >5 μm between two runs.
Typical results
Figure 4 shows a typical Nicomp DLS Method I result. It is worth noting that the chi square calculation is acceptably low at 0.43, and the mean diameter is 313.2 nm, which is well below the 500 nm limit. Figures 5 and 6 exhibit typical AccuSizer Method II results and it is worth noting that the >0.5 μm is considerably larger in the bad result.
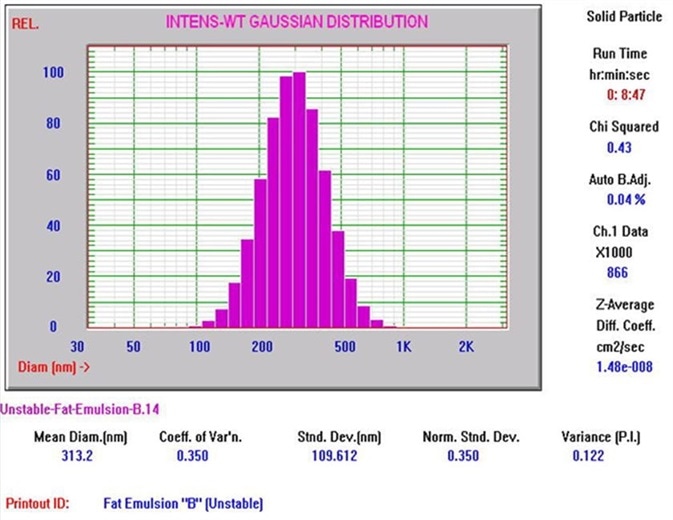
Figure 4. Nicomp DLS Method I result. Image Credit: Entegris Particle Sizing
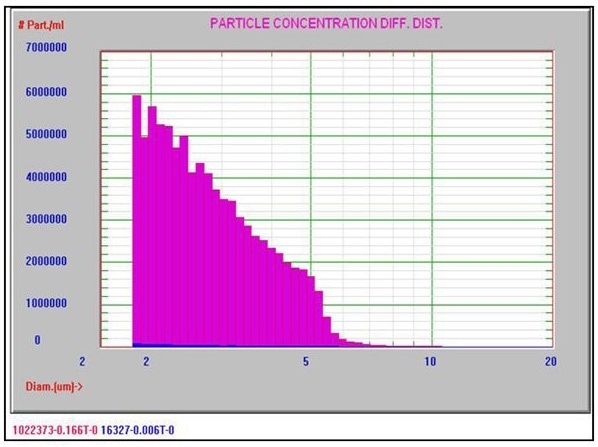
Figure 5. AccuSizer Method II good result. Image Credit: Entegris Particle Sizing
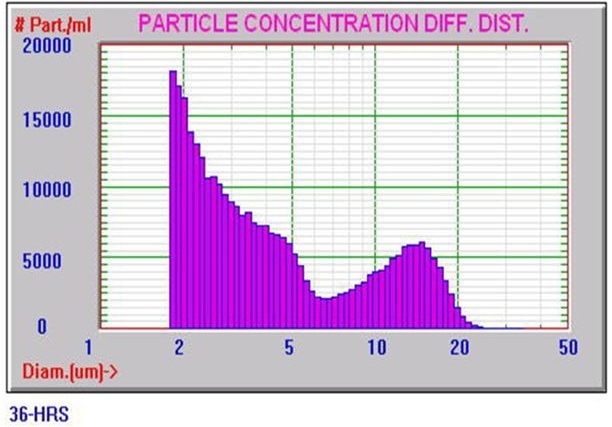
Figure 6. AccuSizer Method II bad result. Image Credit: Entegris Particle Sizing
Conclusions
Entegris supplies the perfect solutions for both Method I, with the Nicomp DLS system, and Method II, with the AccuSizer SPOS system. Due to the highest resolution and best auto dilution features available, the AccuSizer is now the industry standard for Method II tests for the PFAT 5.
About Entegris Particle Sizing
Since our inception in 1978 we have been providing leading edge instrumentation for the field of particle size analysis. We are an applications driven company that provides solutions to our customer's most complex particle sizing and zeta potential monitoring problems. From wet to dry, online to research laboratory we have engineered a complete family of modular designed instruments that can be configured to meet the specific demands of an application.
Single particle optical sizing (SPOS)
The Entegris AccuSizer operates using the principle of single particle optical sizing (SPOS). This technique generates high accuracy, high resolution particle size distribution results as well as concentration data in particles/mL. The AccuSizer platform includes multiple configurations designed to meet a wide array of applications including laboratory and online systems. Multiple sensor options cover a wide range of particle size and concentration limits. Advanced fluidics modules can handle low concentration samples requiring no dilution to highly concentrated inks and CMP slurries using our patented two stage exponential dilution system.
Dynamic light scattering
The Nicomp dynamic light scattering (DLS) system measures particle size below 1 nm and zeta potential to study particle charge. The unique Nicomp algorithm provides the highest resolution results for samples containing more than one peak. Other unique capabilities include auto-dilution and auto-samplers to facilitate high sample throughput.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.