The NObreath® FeNO monitor is a handheld, battery powered, fully portable device that can determine the amount of nitric oxide (NO) exhaled in a person’s breath, often referred to as fractional exhaled nitric oxide (FeNO).
FeNO is used as a biomarker of eosinophilic airway inflammation, as a means to detect and manage various respiratory diseases, especially asthma, which according to Asthma UK, affects more than 5 million adults and children in the UK1.
The recent challenge for Bedfont® Scientific Ltd. has been to get healthcare professionals to implement FeNO testing in general practice. The goal is to cement the NObreath® FeNO monitor as device that can meet all end users’ requirements, while providing an easy solution for physicians to monitor and diagnose many respiratory conditions.
Image credit: Bedfont Scientific
The client
At the University Hospital Llandough, Wales, the Respiratory Unit used the NObreath® FeNO breath monitor to complete 300 tests over a period of five months. In respiratory clinics, FeNO testing serves as an important tool helping consultants both in the diagnosis and differentiation of many respiratory disorders to manage and monitor the patient’s treatment regimen.
Lois Penhaligan, who works as a specialist respiratory physiologist at the Lung Function Laboratory in Llandough, explains the reason for purchasing the FeNO monitor:
The test was being requested by our respiratory consultants during routine clinics with adult patients. This can be used with patients who have respiratory symptoms, particularly with an unexplained cough and we are looking for the possible cause. The FeNO breath test is used for an assessment of airway inflammation, for the assessment of the effectiveness of a treatment, for example, a bronchodilator and for the management of a disease, for example asthma.”
Bedfont’s response
Bedfont® responded by providing a monitor that combines intelligent design and high-quality technology with accessibility and ease of use.
We were looking for a monitor that was lightweight, portable, had a quick analyser to do multiple tests during a very busy respiratory clinic. We chose this particular competitor due to the price of the product which was quoted for the monitor as a whole, in comparison to our previous monitor which had different expenses for different parts of the monitor which also had different expiry dates. We also liked that the monitor did not have an expiry date and the after sales cost was simply to buy more mouthpieces when we ran out. We liked that the monitor was given to us to loan for a period before purchasing.”
Lois Penhaligan, specialist respiratory physiologist at the University Hospital Llandough.
Results
In addition, the NObreath® FeNO testing has considerably enhanced the clinical service at the Llandough respiratory clinic.
It’s a very quick and simple way of measuring airway inflammation. It’s very easy for the patient to use and it is very rare that a patient cannot comply with the technique to produce an accurate result.”
Lois Penhaligan, specialist respiratory physiologist at the University Hospital Llandough.
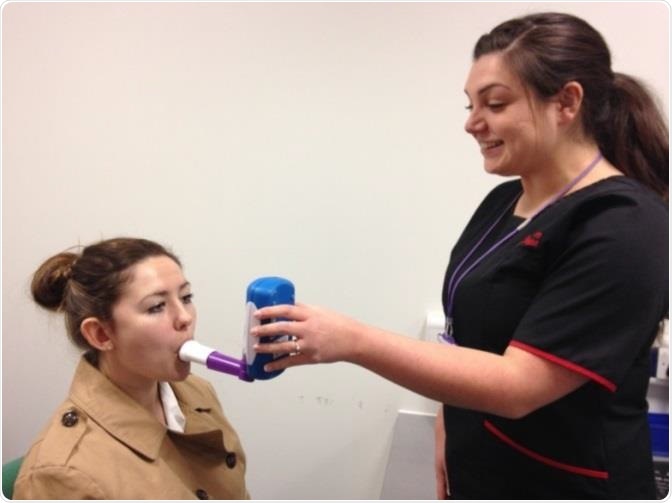
A respiratory physiologist demonstrating the NObreath® FeNO monitor with a student. Image credit: Bedfont Scientific
It is very quick at analysing the breath sample. It has an incentive for the patient to look at during the exhalation in the form of the ball bearing, which helps the patient to gauge the strength of their blow. It is very lightweight and portable meaning it can be moved into different testing rooms with ease. The only downside I feel is that sometimes the mouthpieces are very stiff to get on and will slide off before the patient has put their mouth around or during the test which causes a leak. The ease of pushing on the mouthpiece varies between mouthpieces.
Dr Paul Thomas comments on the NObreath®
A tight seal needs to be achieved between the NObreath® flow and the mouthpiece. It can be easier to join the mouthpiece and the flow together before being inserted into the front of the NObreath® monitor.
Conclusion
The aim for the future is to use the NObreath® and FeNO testing to assist respiratory health care professionals, in both primary and secondary care, toward support of their routine decisions in patient diagnosis and respiratory disorder treatment.
At the University Hospital Llandough, future plans for the Respiratory Unit as cited by Paul Thomas are to:
continue to use the NObreath® as an important departmental equipment tool to helping the process to ascertain both the diagnosis and severity of individual patient’s condition as instructed clinically for our patients by our lead consultant physicians.
Paul Thomas, the head of department and senior chief respiratory physiologist at the Lung Function Laboratory at University of Llandough Hospital, endorsed and agreed the case study work carried out by Lois Penhalighan.
References
- Asthma UK, Asthma facts and statistics. (2017). https://www.asthma.org.uk/about/media/facts-and-statistics/ [accessed 3 January 2017].
About Bedfont Scientific
Bedfont® Scientific has specialised in the design and manufacture of exhaled breath and gas monitoring instruments since 1976.
For medical gas monitoring, their Medi-Gas Check medical pipeline testing range verifies not only the quantity but also quality of gas administered to patients.
Bedfont's breath analysers include carbon monoxide (CO) monitors such as the Smokerlyzer®, used for smoking cessation, and the ToxCO®, used by emergency services, to diagnose CO poisoning.
The NObreath® FeNO monitor provides accurate analysis of airway inflammation for the control of asthma, and the Gastrolyzer® range aids in the detection of gastrointestinal disorders and food intolerances. Quick and non-invasive, breath analysis is the new blood test.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.